acid base K. K, name formula name formula ethylamine C2H5NH, 6.4× 10 hydrofluoric acid HF 6.8 × 10¬4 -4 HCH,CO2 1.8 × 10¯³ NH3 1.8 x 10 acetic acid ammonia Use this data to rank the following solutions in order of increasing pH. In other words, select a '1' next to the solution that will have the lowest pH, a '2' next to the solution that will have the next lowest pH, and so on. solution pH 0.1 M NaF choose one v 0.1 M KCH3CO2 choose onev 0.1 M NH4CI choose onev 0.1 M NANO3 choose onev
acid base K. K, name formula name formula ethylamine C2H5NH, 6.4× 10 hydrofluoric acid HF 6.8 × 10¬4 -4 HCH,CO2 1.8 × 10¯³ NH3 1.8 x 10 acetic acid ammonia Use this data to rank the following solutions in order of increasing pH. In other words, select a '1' next to the solution that will have the lowest pH, a '2' next to the solution that will have the next lowest pH, and so on. solution pH 0.1 M NaF choose one v 0.1 M KCH3CO2 choose onev 0.1 M NH4CI choose onev 0.1 M NANO3 choose onev
Chapter14: Acids And Bases
Section: Chapter Questions
Problem 10RQ: For oxyacids, how does acid strength depend on a. the strength of the bond to the acidic hydrogen...
Related questions
Question
Please rank from 1 (lowest) to 4 (highest). Thank you!
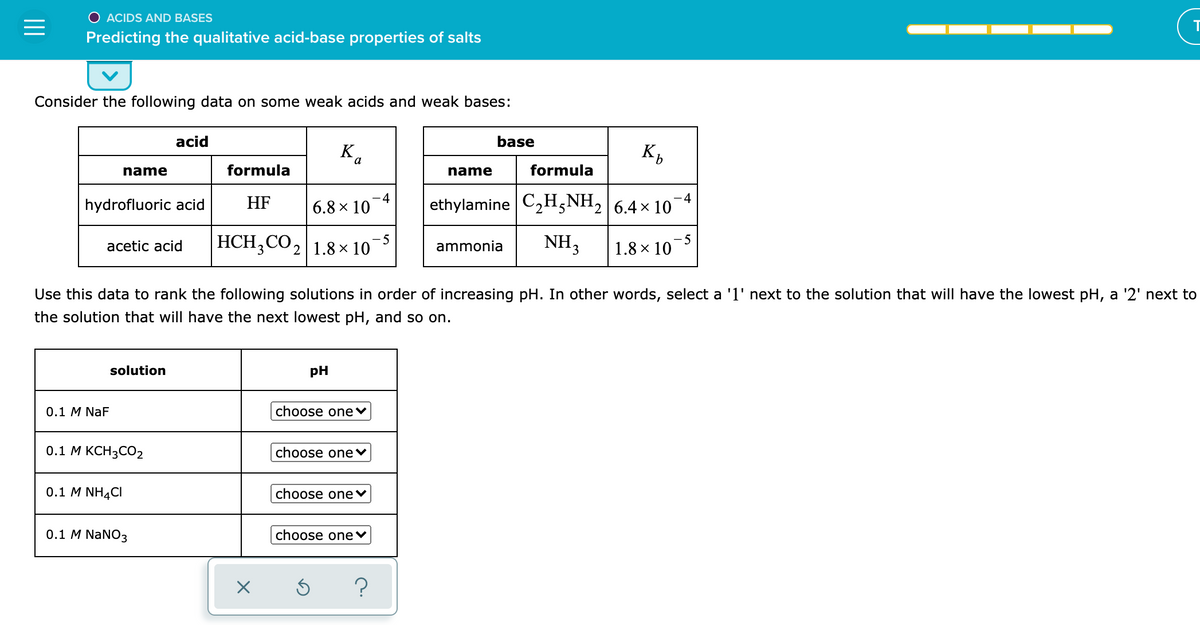
Transcribed Image Text:O ACIDS AND BASES
Predicting the qualitative acid-base properties of salts
Consider the following data on some weak acids and weak bases:
acid
base
K.
a
name
formula
name
formula
-4
-4
hydrofluoric acid
HF
6.8 x 10
ethylamine C2H,NH, 6.4 x 10
HCH;CO, 1.8 × 10
-5
acetic acid
ammonia
NH,
-5
1.8 × 10
Use this data to rank the following solutions in order of increasing pH. In other words, select a '1' next to the solution that will have the lowest pH, a '2' next to
the solution that will have the next lowest pH, and so on.
solution
pH
0.1 M NaF
choose one♥
0.1 М КСН3СО2
choose onev
0.1 M NH4CI
choose one♥
0.1 M NaNO3
choose one♥
?
II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning