Consider the following data on some weak acids and weak bases: acid base K. K, name formula name formula -4 hypochlorous acid HCIO 3.0 x 10-8 methylamine CH,NH, 4.4 x 10 hydrocyanic acid НCN -10 4.9 x 10 NH3 -5 1.8 × 10 ammonia Use this data to rank the following solutions in order of increasing pH. In other words, select a 'l' next to the solution that will have the lowest the solution that will have the next lowest pH, and so on. solution pH 0.1 M CH3NH3CI choose onev 0.1 M NaCN choose one v 0.1 М KCIO choose onev 0.1 M Nal choose one v
Consider the following data on some weak acids and weak bases: acid base K. K, name formula name formula -4 hypochlorous acid HCIO 3.0 x 10-8 methylamine CH,NH, 4.4 x 10 hydrocyanic acid НCN -10 4.9 x 10 NH3 -5 1.8 × 10 ammonia Use this data to rank the following solutions in order of increasing pH. In other words, select a 'l' next to the solution that will have the lowest the solution that will have the next lowest pH, and so on. solution pH 0.1 M CH3NH3CI choose onev 0.1 M NaCN choose one v 0.1 М KCIO choose onev 0.1 M Nal choose one v
Chapter14: Acids And Bases
Section: Chapter Questions
Problem 10RQ: For oxyacids, how does acid strength depend on a. the strength of the bond to the acidic hydrogen...
Related questions
Question
1 (lowest) to 4 (highest)
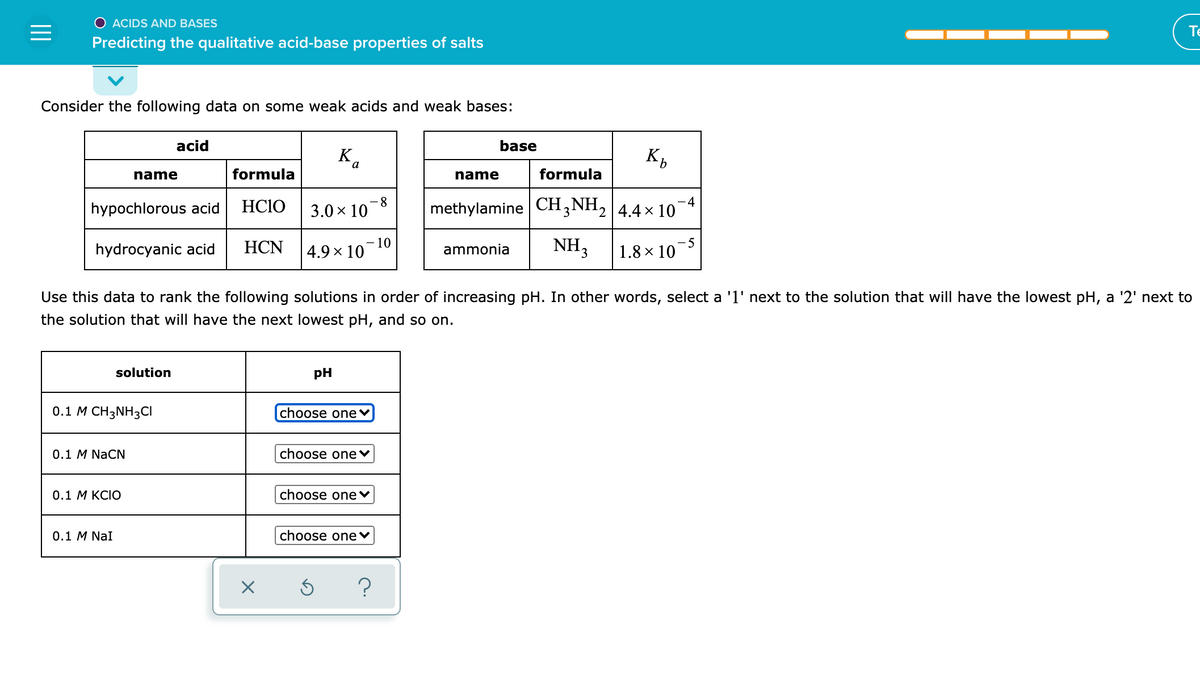
Transcribed Image Text:O ACIDS AND BASES
Te
Predicting the qualitative acid-base properties of salts
Consider the following data on some weak acids and weak bases:
acid
base
Ka
name
formula
name
formula
8.
3.0 x 10
hypochlorous acid
HC1O
methylamine CH3 NH2 4.4× 10
- 4
hydrocyanic acid
HCN
10
4.9 x 10
NH3
- 5
1.8 x 10
ammonia
Use this data to rank the following solutions in order of increasing pH. In other words, select a '1' next to the solution that will have the lowest pH, a '2' next to
the solution that will have the next lowest pH, and so on.
solution
pH
0.1 M CH3NH3CI
choose one♥
0.1 M NaCN
choose onev
0.1 М KCIO
choose onev
0.1 M NaI
choose one♥
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning