acids: O 0.11 mol of HCI is added to 1.0 L of a 1.0M NH, bases: U solution. other: O 0.44 mol of NaOH is added acids: U to 1.0 L of a solution that is 1.1M in both NH, and bases: U NH CI. O other: U
acids: O 0.11 mol of HCI is added to 1.0 L of a 1.0M NH, bases: U solution. other: O 0.44 mol of NaOH is added acids: U to 1.0 L of a solution that is 1.1M in both NH, and bases: U NH CI. O other: U
ChapterU6: Showtime: Reversible Reactions And Chemical Equilibrium
Section: Chapter Questions
Problem 17STP
Related questions
Question
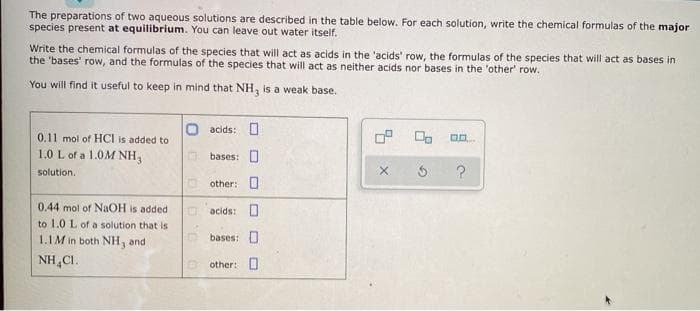
Transcribed Image Text:The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major
species present at equilibrium. You can leave out water itself.
Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in
the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row.
You will find it useful to keep in mind that NH, is a weak base.
acids: O
0.11 mol of HCI is added to
1.0 L of a 1.0M NH3
bases: 0
solution.
other: O
0.44 mol of NAOH is added
acids: O
to 1.0 L of a solution that is
1.1M in both NH, and
bases: O
NH,CI.
O other: O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER



Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning