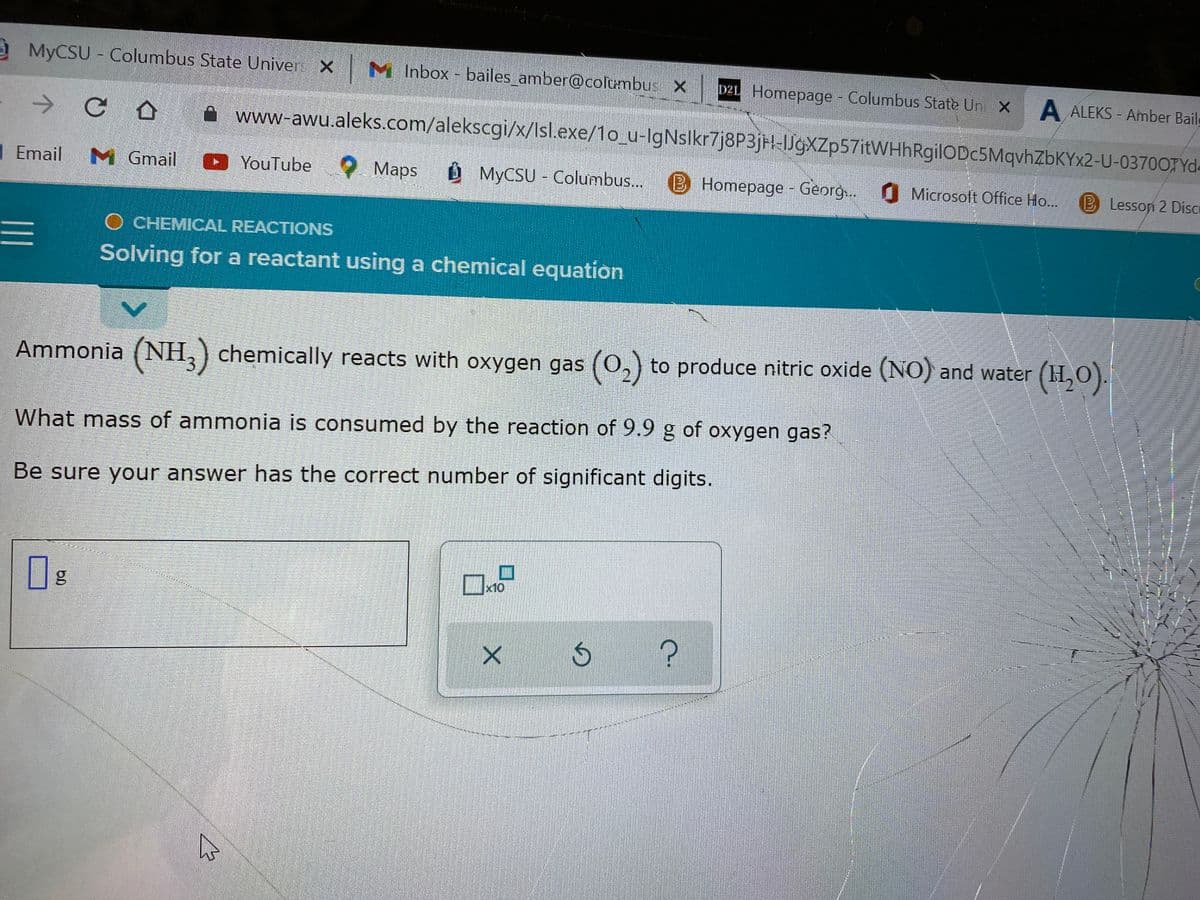
Ammonia (NH3) chemically reacts with oxygen gas (0₂) to produce nitric oxide (NO) and water (H₂O). What mass of ammonia is consumed by the reaction of 9.9 g of oxygen gas? Be sure your answer has the correct number of significant digits. 0
Ammonia (NH3) chemically reacts with oxygen gas (0₂) to produce nitric oxide (NO) and water (H₂O). What mass of ammonia is consumed by the reaction of 9.9 g of oxygen gas? Be sure your answer has the correct number of significant digits. 0
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter11: Solutions
Section: Chapter Questions
Problem 60P
Related questions
Question
Transcribed Image Text:MyCSU - Columbus State Univer. x | № Inbox - bailes_amber@columbu: x D2L Homepage - Columbus State Un X
- со
A ALEKS-Amber Bail
www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-lJgXZp57itWHhRgilODc5MqvhZbKYx2-U-037007TYd
Gmail
YouTube
Maps
MyCSU - Columbus... Homepage - Georg... Microsoft Office Ho... B Lesson 2 Disc
O CHEMICAL REACTIONS
=
Solving for a reactant using a chemical equation
Ammonia (NH3) chemically reacts with oxygen gas (0₂) to produce nitric oxide (NO) and water (1₂0).
What mass of ammonia is consumed by the reaction of 9.9 g of oxygen gas?
Be sure your answer has the correct number of significant digits.
x10
g
ANAKKALE
X
S
?
Email
4
Jessy
Vseforestainty a jedan den so
$45******
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning