Ammonium dichromate is sometimes known as Vesuvian Fire, because of its use in demonstrations of tabletop "volcanoes". Using the given data, calculate the heat of reaction for the following reaction: (NH4)2Cr207(s) → Cr2O3(s) + N2(g) +4H2O(g) s° ΔΗΡ (k) mol") (U mol- K-') AG (k) mol) Species Nitrogen N;(g) 191.5 N(g) NH,(g) 472.704 153.19 455.579 -46.11 192.3 -16.5 NH,(aq) NH,*(aq) N,H,(() (NH,),AsO,(aq) -80.29 111.3 -26.50 -132.51 113.4 -79.31 50.63 121.2 149.2 -1268 NH,Br(s) -270.83 113 -175.2 NH,CI(s) NH,CI(aq) -314.4 94.6 -201.5 - 300.2
Ammonium dichromate is sometimes known as Vesuvian Fire, because of its use in demonstrations of tabletop "volcanoes". Using the given data, calculate the heat of reaction for the following reaction: (NH4)2Cr207(s) → Cr2O3(s) + N2(g) +4H2O(g) s° ΔΗΡ (k) mol") (U mol- K-') AG (k) mol) Species Nitrogen N;(g) 191.5 N(g) NH,(g) 472.704 153.19 455.579 -46.11 192.3 -16.5 NH,(aq) NH,*(aq) N,H,(() (NH,),AsO,(aq) -80.29 111.3 -26.50 -132.51 113.4 -79.31 50.63 121.2 149.2 -1268 NH,Br(s) -270.83 113 -175.2 NH,CI(s) NH,CI(aq) -314.4 94.6 -201.5 - 300.2
Chapter9: Energy For Today
Section: Chapter Questions
Problem 9E
Related questions
Question
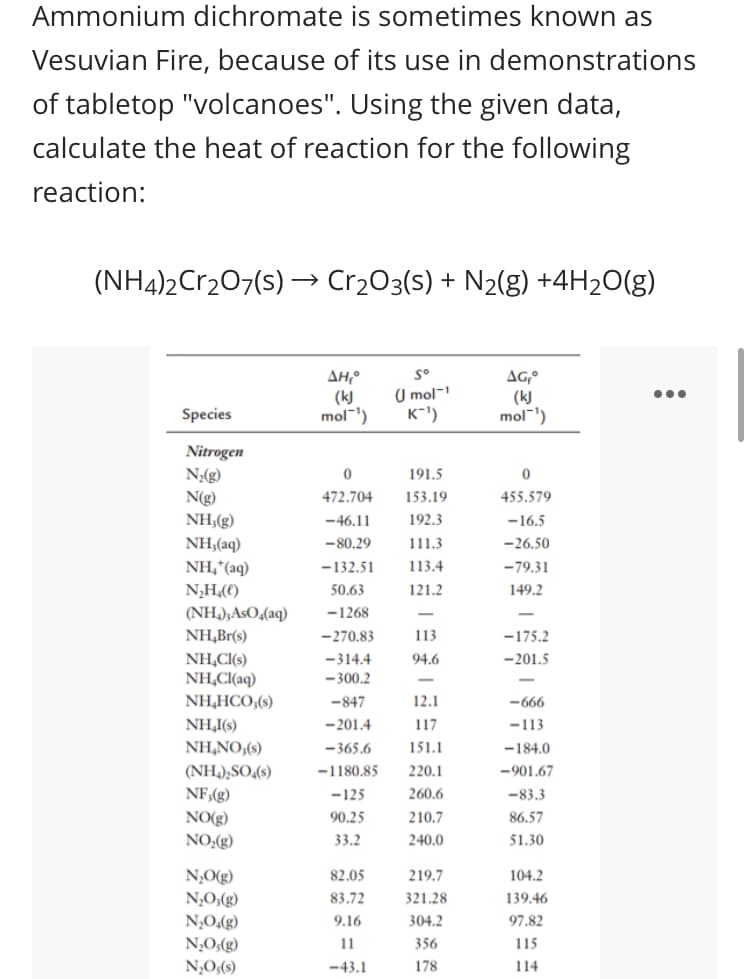
Transcribed Image Text:Ammonium dichromate is sometimes known as
Vesuvian Fire, because of its use in demonstrations
of tabletop "volcanoes". Using the given data,
calculate the heat of reaction for the following
reaction:
(NH4)2Cr207(s) → Cr2O3(s) + N2(g) +4H2O(g)
AH,°
(kJ
mol")
s°
U mol-
K-')
(k)
mol')
Species
Nitrogen
N;(g)
N(g)
NH,(g)
191.5
472.704
153.19
455.579
-46.11
192.3
-16.5
NH,(aq)
NH,*(aq)
-80.29
111.3
-26.50
-132.51
113.4
-79.31
N,H(€)
50.63
121.2
149.2
(NH,);AsO,(aq)
NH,Br(s)
-1268
-270.83
113
-175.2
NH,C(s)
NH,Cl(aq)
NH,HCO,(s)
-314.4
94.6
-201.5
- 300.2
-847
12.1
-666
- 201.4
NH,I(s)
NH,NO,(s)
117
-113
- 365.6
151.1
-184.0
(NH,);SO,(s)
-1180.85
220.1
-901.67
NF,(g)
-125
260.6
-83.3
210.7
86.57
NO(g)
NO:(g)
90.25
33.2
240.0
51.30
N,O(g)
82.05
219.7
104.2
83.72
321.28
139.46
(3)'O°N
304.2
N,O,(g)
9.16
97.82
N;O;(g)
N,O;(s)
11
356
115
-43.1
178
114
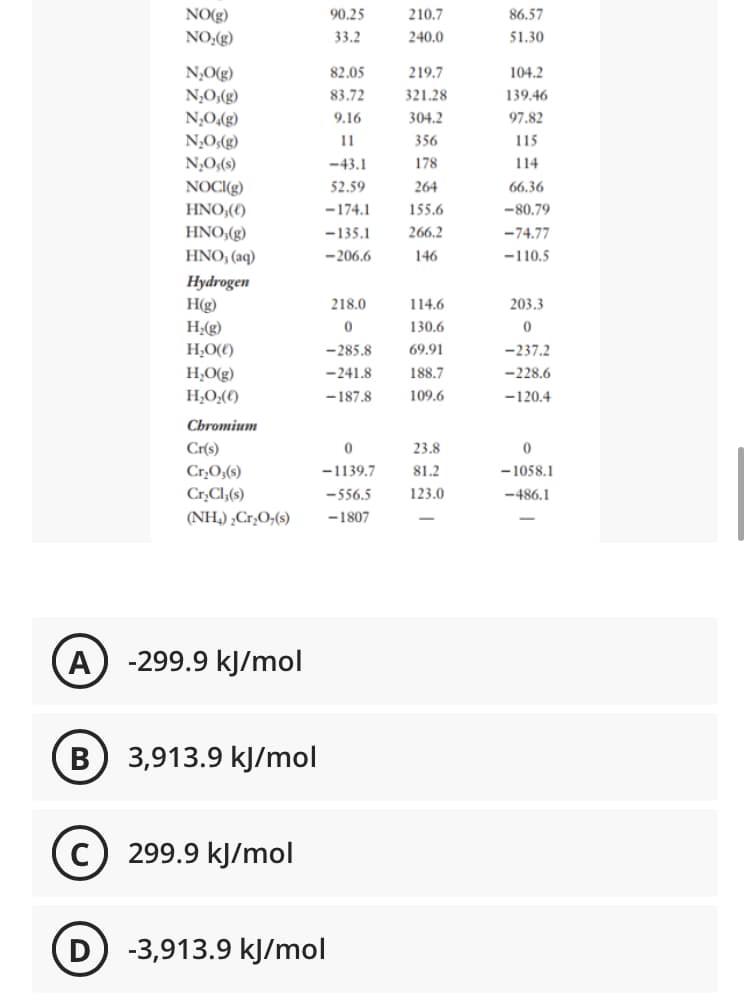
Transcribed Image Text:NO(g)
NO;(g)
90.25
210.7
86.57
33.2
240.0
51.30
N,O(g)
N;O,(g)
82.05
219.7
104.2
83.72
321.28
139.46
(S)*O°N_
(3)*O°N
9.16
304.2
97.82
11
356
115
N,O;(s)
-43.1
178
114
NOCI(g)
HNO,(€)
HNO,(g)
52.59
264
66,36
-174.1
155.6
-80.79
-135.1
266.2
-74.77
HNO, (aq)
-206.6
146
-110.5
Hydrogen
H(g)
218.0
114.6
203.3
H;(g)
130.6
H,O(€)
-285.8
69.91
-237.2
H,O(g)
-241.8
188.7
-228.6
H;O;()
-187.8
109.6
-120.4
Cbromium
Cr(s)
23.8
Cr,O;(s)
Cr,Cl;(s)
-1139.7
81.2
- 1058.1
-556.5
123.0
-486.1
(NH,) ¿Cr,O;(s)
-1807
A
-299.9 kJ/mol
В
3,913.9 kJ/mol
299.9 kJ/mol
D
-3,913.9 kJ/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning