Ammonium perchlorate (NH,CIO,) is a powerful solid rocket fuel, used in the Space Shuttle boosters. It decomposes into nitrogen (N,) gas, chlorine (Cl,) gas, oxygen (0,) gas and water vapor, releasing a great deal of energy. Calculate the moles of water produced by the reaction of 2.0 mol of ammonium perchlorate. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.
Ammonium perchlorate (NH,CIO,) is a powerful solid rocket fuel, used in the Space Shuttle boosters. It decomposes into nitrogen (N,) gas, chlorine (Cl,) gas, oxygen (0,) gas and water vapor, releasing a great deal of energy. Calculate the moles of water produced by the reaction of 2.0 mol of ammonium perchlorate. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter2: Chemical Formulas, Equations, And Reaction Yields
Section: Chapter Questions
Problem 16P: At its boiling point (280°C) and at atmospheric pressure, phosphorus has a gas density of 2.7gL1 ....
Related questions
Question
19
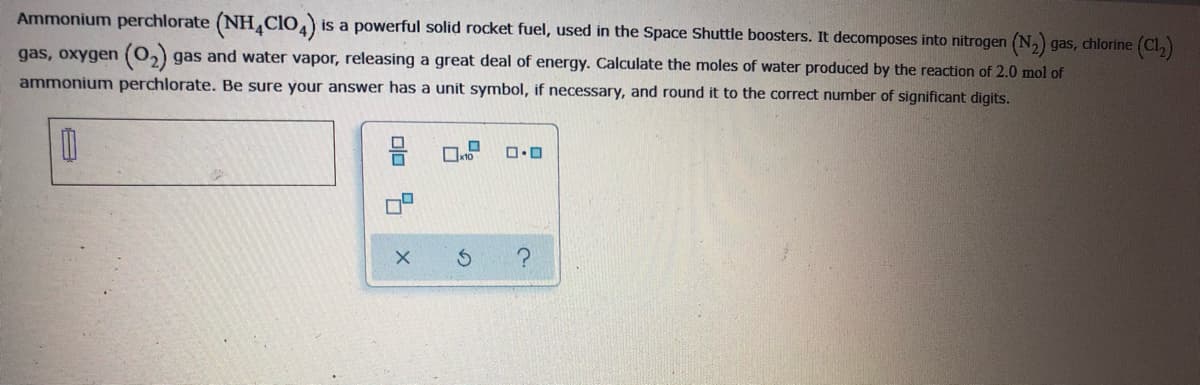
Transcribed Image Text:Ammonium perchlorate (NH,CIO,)
is a powerful solid rocket fuel, used in the Space Shuttle boosters. It decomposes into nitrogen (N,) gas, chlorine (Cl,)
gas, oxygen (0,) gas and water vapor, releasing a great deal of energy. Calculate the moles of water produced by the reaction of 2.0 mol of
ammonium perchlorate. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.
olo 5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning
