Ammonium phosphate ((NH),PO) is an important ingredient in many fertilizers. It can be made by reacting phosphoric acid (H,PO,) with ammonia ( What mass of ammonium phosphate is produced by the reaction of 4.35 g of phosphoric acid? Round your answer to 3 significant digits. 0. D.P 2
Ammonium phosphate ((NH),PO) is an important ingredient in many fertilizers. It can be made by reacting phosphoric acid (H,PO,) with ammonia ( What mass of ammonium phosphate is produced by the reaction of 4.35 g of phosphoric acid? Round your answer to 3 significant digits. 0. D.P 2
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 101AE: This year, like many past years, you begin to feel very sleepy alter eating a large helping of...
Related questions
Question
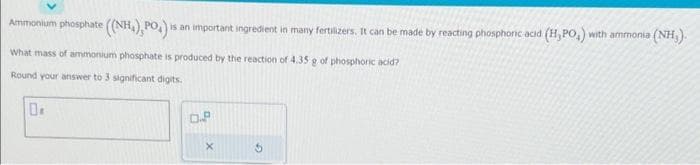
Transcribed Image Text:Ammonium phosphate ((NH), PO₂) =
is an important ingredient in many fertilizers. It can be made by reacting phosphoric acid (H,PO,) with ammonia (NH₂).
What mass of ammonium phosphate is produced by the reaction of 4.35 g of phosphoric acid?
Round your answer to 3 significant digits.
0.2
G
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
