An 8.0L tank at 29*C is filled with 16.6g carbon monoxide gas and 6.5g of sulfur hexafluoride gas. You can assume both gases behave ad ideal gases under these conditions. Calculate the mold fraction of each gas. Round your answer to 3 significant digits.
An 8.0L tank at 29*C is filled with 16.6g carbon monoxide gas and 6.5g of sulfur hexafluoride gas. You can assume both gases behave ad ideal gases under these conditions. Calculate the mold fraction of each gas. Round your answer to 3 significant digits.
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter5: Gases
Section: Chapter Questions
Problem 5.93PAE: 93 The complete combustion of octane can be used as a model for the burning of gasoline:...
Related questions
Question
An 8.0L tank at 29*C is filled with 16.6g carbon monoxide gas and 6.5g of sulfur hexafluoride gas. You can assume both gases behave ad ideal gases under these conditions. Calculate the mold fraction of each gas. Round your answer to 3 significant digits.
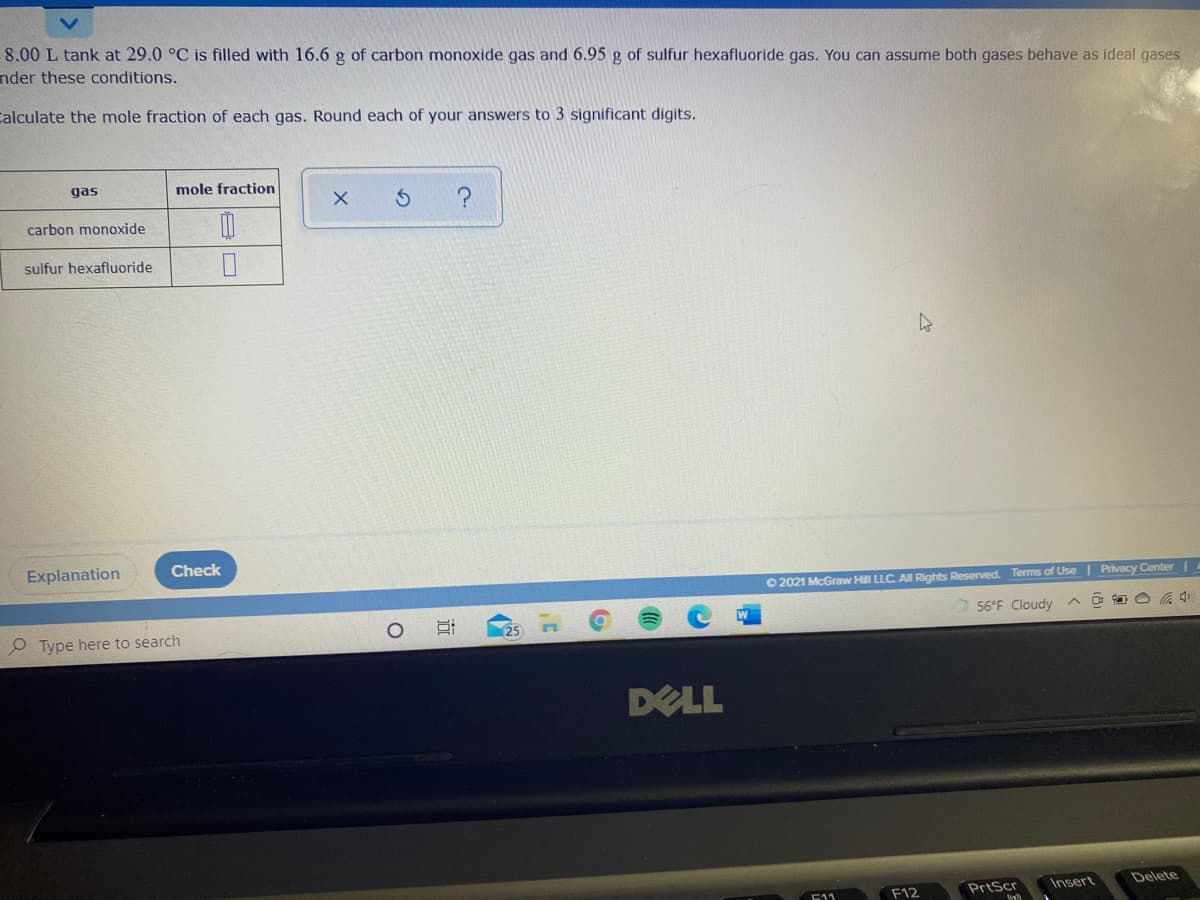
Transcribed Image Text:8.00 L tank at 29.0 °C is filled with 16.6 g of carbon monoxide gas and 6.95 g of sulfur hexafluoride gas. You can assume both gases behave as ideal gases
nder these conditions.
Calculate the mole fraction of each gas. Round each of your answers to 3 significant digits.
gas
mole fraction
carbon monoxide
sulfur hexafluoride
Explanation
Check
O 2021 McGraw Hill LLC. AII Rights Reserved. Terms of Use | Privacy Center |
56°F Cloudy
25
O Type here to search
DELL
Insert
Delete
F12
PrtScr
511
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning