An analytical chemist is titrating 83.7 mL of a 0.3300M solution of isopropylamine ((CH,) CHNH,) with a 0.5300M solution of HNO3. The p K, of isopropylamine is 3.33. Calculate the pH of the base solution after the chemist has added 42.4 mL of the HNO, solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO, solution added. Round your answer to 2 decimal places. pH = ?
An analytical chemist is titrating 83.7 mL of a 0.3300M solution of isopropylamine ((CH,) CHNH,) with a 0.5300M solution of HNO3. The p K, of isopropylamine is 3.33. Calculate the pH of the base solution after the chemist has added 42.4 mL of the HNO, solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO, solution added. Round your answer to 2 decimal places. pH = ?
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter15: Acid–base Equilibria
Section: Chapter Questions
Problem 55P
Related questions
Question
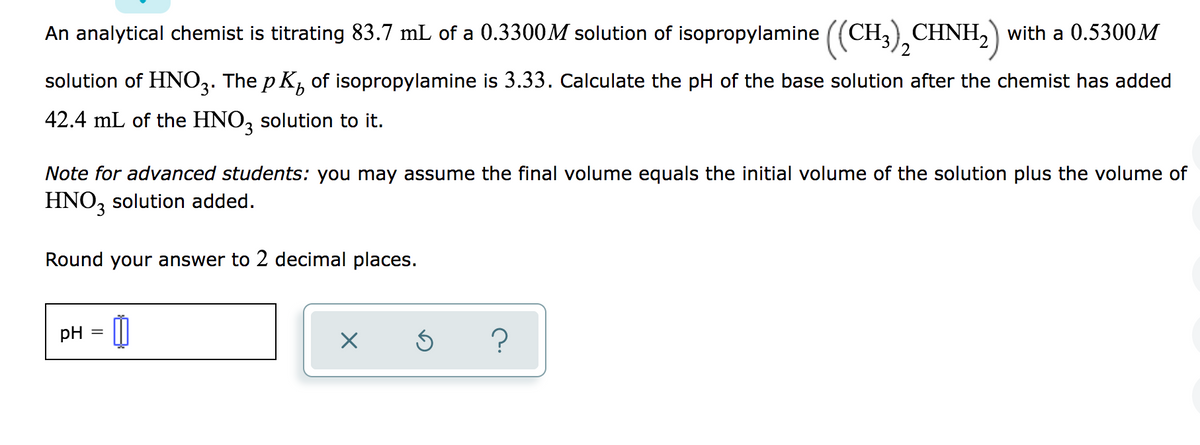
Transcribed Image Text:An analytical chemist is titrating 83.7 mL of a 0.3300M solution of isopropylamine ((CH,) CHNH, with a 0.5300M
2
solution of HNO3. The p K, of isopropylamine is 3.33. Calculate the pH of the base solution after the chemist has added
42.4 mL of the HNO, solution to it.
Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of
HNO, solution added.
Round your answer to 2 decimal places.
pH = |
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning