An equimolar blend of 1,4-hydroquinone diallylether and ethylene glycol dimercaptoacetate containing a small amount of benzophenone (radical photoinitiator) is polymerized by irradiating the blend with ultraviolet (UV) light. (a) Write a balanced chemical equation for the polymerization. (b) How would you classify this polymerization according to the reaction mechanism? (c) Calculate the extent of reaction needed to produce a polymer with a number average molar mass of 20,000 g/mole (neglect the effect of end groups in the calculation). (d) Draw the structure of the polymer that is obtained when the mole ratio of diallyl ether to dithiol is 1.1. (e) Explain briefly the consequences of including a tetrafunctional thiol in the polymerization with the exact stoichiometry 2:1:4 of difunctional thiol to tetrafunctional thiol to difunctional allylic ether. SH HS 1,4-hydroquinone diallylether Ethylene glycol dimercaptoacetate
An equimolar blend of 1,4-hydroquinone diallylether and ethylene glycol dimercaptoacetate containing a small amount of benzophenone (radical photoinitiator) is polymerized by irradiating the blend with ultraviolet (UV) light. (a) Write a balanced chemical equation for the polymerization. (b) How would you classify this polymerization according to the reaction mechanism? (c) Calculate the extent of reaction needed to produce a polymer with a number average molar mass of 20,000 g/mole (neglect the effect of end groups in the calculation). (d) Draw the structure of the polymer that is obtained when the mole ratio of diallyl ether to dithiol is 1.1. (e) Explain briefly the consequences of including a tetrafunctional thiol in the polymerization with the exact stoichiometry 2:1:4 of difunctional thiol to tetrafunctional thiol to difunctional allylic ether. SH HS 1,4-hydroquinone diallylether Ethylene glycol dimercaptoacetate
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter29: Organic Polymer Chemistry
Section: Chapter Questions
Problem 29.8P
Related questions
Question
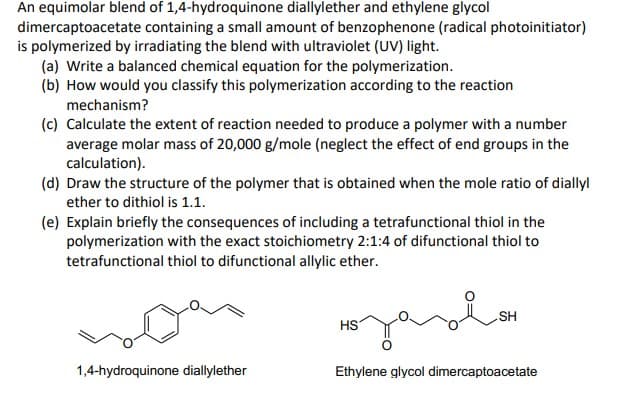
Transcribed Image Text:An equimolar blend of 1,4-hydroquinone diallylether and ethylene glycol
dimercaptoacetate containing a small amount of benzophenone (radical photoinitiator)
is polymerized by irradiating the blend with ultraviolet (UV) light.
(a) Write a balanced chemical equation for the polymerization.
(b) How would you classify this polymerization according to the reaction
mechanism?
(c) Calculate the extent of reaction needed to produce a polymer with a number
average molar mass of 20,000 g/mole (neglect the effect of end groups in the
calculation).
(d) Draw the structure of the polymer that is obtained when the mole ratio of diallyl
ether to dithiol is 1.1.
(e) Explain briefly the consequences of including a tetrafunctional thiol in the
polymerization with the exact stoichiometry 2:1:4 of difunctional thiol to
tetrafunctional thiol to difunctional allylic ether.
HS
HS
1,4-hydroquinone diallylether
Ethylene glycol dimercaptoacetate
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning
