An imaginary element Z has two naturally occurring isotopes, ³Z and 4Z. ³Z has a mass of 3.016 amu, and "Z has a mass of 4.003 amu. The atomic mass for the element Z is 3.878 amu. What is the percent abundance of 4Z?
An imaginary element Z has two naturally occurring isotopes, ³Z and 4Z. ³Z has a mass of 3.016 amu, and "Z has a mass of 4.003 amu. The atomic mass for the element Z is 3.878 amu. What is the percent abundance of 4Z?
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter2: Atoms, Molecules, And Ions
Section: Chapter Questions
Problem 2.136QE
Related questions
Question
please answer question 1
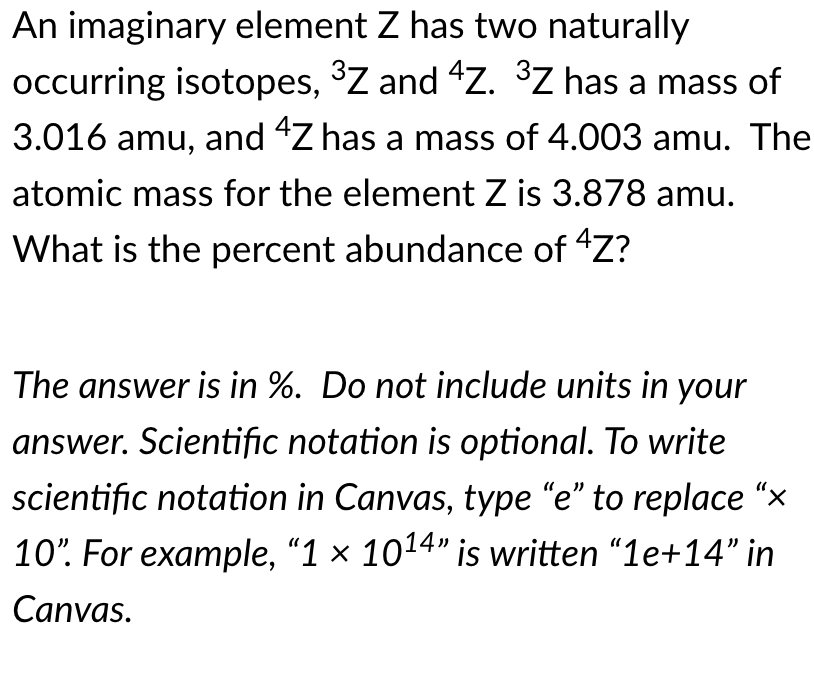
Transcribed Image Text:An imaginary element Z has two naturally
occurring isotopes, ³Z and 4Z. ³Z has a mass of
3.016 amu, and "Z has a mass of 4.003 amu. The
atomic mass for the element Z is 3.878 amu.
What is the percent abundance of "Z?
The answer is in %. Do not include units in your
answer. Scientific notation is optional. To write
scientific notation in Canvas, type "e" to replace “x
10". For example, "1 × 1014" is written "1e+14" in
Canvas.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning