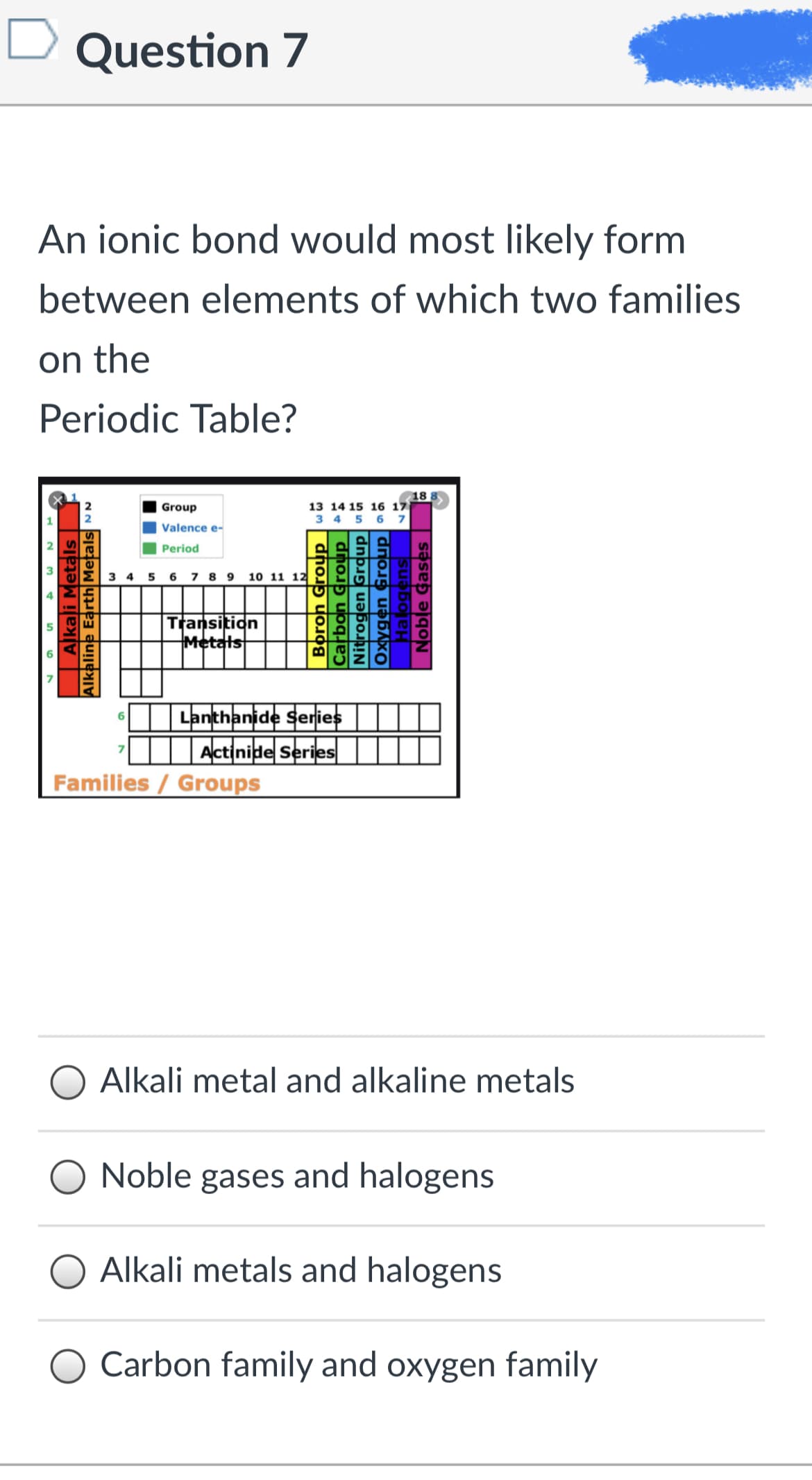
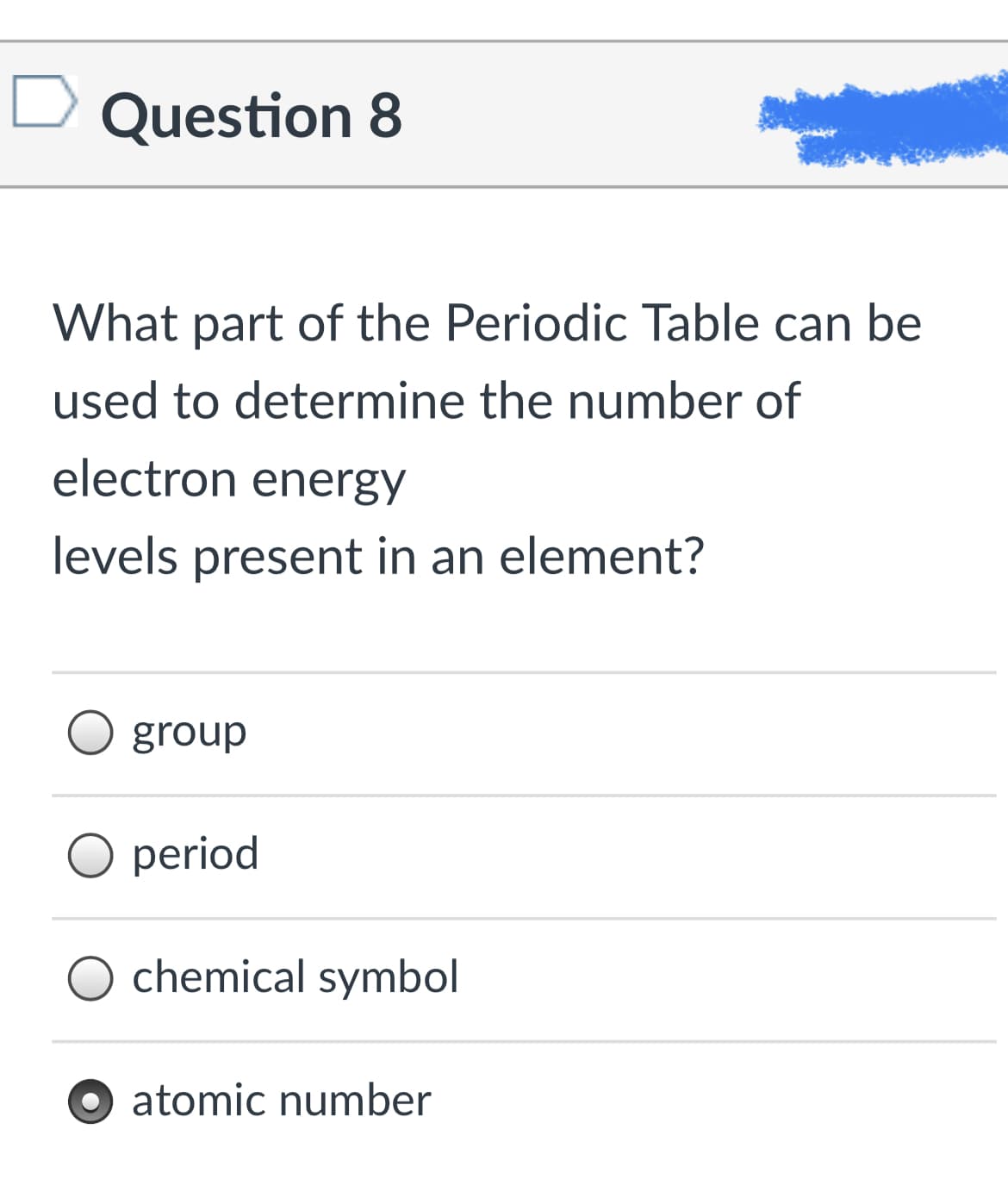
An ionic bond would most likely form between elements of which two families on the Periodic Table? 18 13 14 15 16 17 3 4 5 6 7 Group Valence e- Period 3 4 5 6 7 8 9 10 11 12 Transition Metals Lanthanide series Actinide Series Families / Groups 6 Alkali metal and alkaline metals Noble gases and halogens Alkali metals and halogens Carbon family and oxygen family Alkali Metals Alkaline Earth Metals Boron Group Carbon Group Nitrogen Grdup un Oxygen Group Halogens Noble Gases|
Types of Chemical Bonds
The attractive force which has the ability of holding various constituent elements like atoms, ions, molecules, etc. together in different chemical species is termed as a chemical bond. Chemical compounds are dependent on the strength of chemical bonds between its constituents. Stronger the chemical bond, more will be the stability in the chemical compounds. Hence, it can be said that bonding defines the stability of chemical compounds.
Polarizability In Organic Chemistry
Polarizability refers to the ability of an atom/molecule to distort the electron cloud of neighboring species towards itself and the process of distortion of electron cloud is known as polarization.
Coordinate Covalent Bonds
A coordinate covalent bond is also known as a dative bond, which is a type of covalent bond. It is formed between two atoms, where the two electrons required to form the bond come from the same atom resulting in a semi-polar bond. The study of coordinate covalent bond or dative bond is important to know about the special type of bonding that leads to different properties. Since covalent compounds are non-polar whereas coordinate bonds results always in polar compounds due to charge separation.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps









