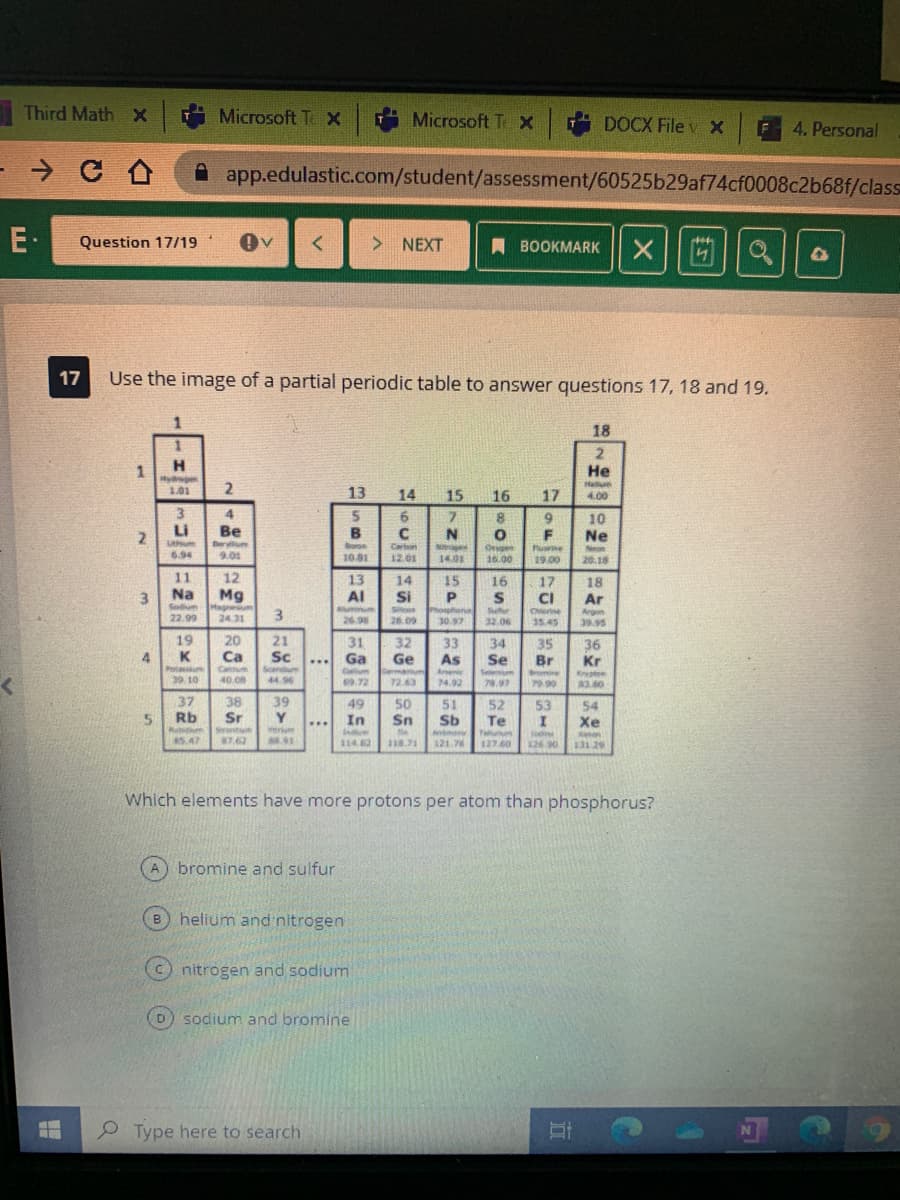
17 Use the image of a partial periodic table to answer questions 17, 18 and 19. 18 H. Hydgen 101 1. Не Hellum 13 14 15 16 17 4.00 3. 4. 5. B 6 C 7. 8. Li 2. 10 Ne Be N Ber 9.01 Borse 10.81 Carbon Oen 16.00 Puine 6.94 12.01 14.01 19.00 20.18 11 12 13 Al 14 15 16 17 18 Ar Na Mg 3. 3 Si CI Sodum Sufer Chere Argen 39.95 22.99 24.31 26.98 26.09 30.97 32.06 35.45 19 K 20 Ca 21 31 Ga 32 Ge 33 Sc 34 Se 35 Br 36 Kr 4 As ... Calum Ae 39. 10 40.08 44.96 09.72 72.63 74.92 78.97 79.90 3.60 37 Rb 38 Sr 39 Y inerium 49 In 50 Sn 51 Sb 52 Te Taurum 127.60 53 54 Xe ... Srontum 5.47 87.62 114 82 118.71 121 76 26 90 13129 Which elements have more protons per atom than phosphorus? A bromine and sulfur helium and nitrogen B. nitrogen and sodium sodium and bromine 2.
17 Use the image of a partial periodic table to answer questions 17, 18 and 19. 18 H. Hydgen 101 1. Не Hellum 13 14 15 16 17 4.00 3. 4. 5. B 6 C 7. 8. Li 2. 10 Ne Be N Ber 9.01 Borse 10.81 Carbon Oen 16.00 Puine 6.94 12.01 14.01 19.00 20.18 11 12 13 Al 14 15 16 17 18 Ar Na Mg 3. 3 Si CI Sodum Sufer Chere Argen 39.95 22.99 24.31 26.98 26.09 30.97 32.06 35.45 19 K 20 Ca 21 31 Ga 32 Ge 33 Sc 34 Se 35 Br 36 Kr 4 As ... Calum Ae 39. 10 40.08 44.96 09.72 72.63 74.92 78.97 79.90 3.60 37 Rb 38 Sr 39 Y inerium 49 In 50 Sn 51 Sb 52 Te Taurum 127.60 53 54 Xe ... Srontum 5.47 87.62 114 82 118.71 121 76 26 90 13129 Which elements have more protons per atom than phosphorus? A bromine and sulfur helium and nitrogen B. nitrogen and sodium sodium and bromine 2.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1ALQ: Paracelsus, a sixteenth-century alchemist and healer, adopted as his slogan: The patients are your...
Related questions
Question
Transcribed Image Text:Third Math X
Microsoft T x
Microsoft Te x
DOCX File v X
4. Personal
- → C A
app.edulastic.com/student/assessment/60525b29af74cf0008c2b68f/class
E-
> NEXT
Question 17/19
A BOOKMARK
17
Use the image of a partial periodic table to answer questions 17, 18 and 19.
18
2
Не
H.
1
Hyder
2
Hellum
4.00
101
13
14
15
16
17
4.
6.
C
8
10
Ne
2.
Li
Be
B
N
F
Lthum
694
Berylum
Borge
Carton
10.81
1
12.01
Devgen
16.00
Puine
Neon
20.18
9.01
14.01
19.00
11
12
13
14
15
16
17
18
Ar
Na
Mg
sum
24.31
Al
CI
3.
Sodum
22.99
Si
See
26.09
Aumin
Argon
39.95
Cherine
3.
30.97
26.98
32.06
15 45
19
K
31
20
21
32
Ge
33
As
34
Ca
Sc
35
Br
36
Kr
Ga
Caum
69.72
Se
...
Protacsum
39. 10
Caldum
40.08
Arsenir
Sele
Bromine
44.96
72.63
74.92
78.97
79 90
3.80
38
37
Rb
39
Y.
49
50
Sn
51
Sb
52
53
54
Sr
In
Te
Xe
...
Srentium
erum
due
114.82
emony
Taluum
Jodine
5.47
87.62
118.71
121.76
127.60
126.90
131.29
Which elements have more protons per atom than phosphorus?
bromine and sulfur
B helium and nitrogen
nitrogen and sodium
sodium and bromine
Type here to search
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

