Analysis: 1. Reconcile your prediction with your observations. Write a definition for limiting and excess reactants based on your observations. 2. Calculate the number of moles of the excess reactant that is left over in each of the reaction trials: Trial 1: Trial 2: Trial 3: I
Analysis: 1. Reconcile your prediction with your observations. Write a definition for limiting and excess reactants based on your observations. 2. Calculate the number of moles of the excess reactant that is left over in each of the reaction trials: Trial 1: Trial 2: Trial 3: I
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter2: Chemical Formulas, Equations, And Reaction Yields
Section: Chapter Questions
Problem 38P: Titanium dioxide, TiO2 , reacts with carbon and chlorineto give gaseous TiCl4 :...
Related questions
Question
Qs 1 and 2
Transcribed Image Text:es)
80
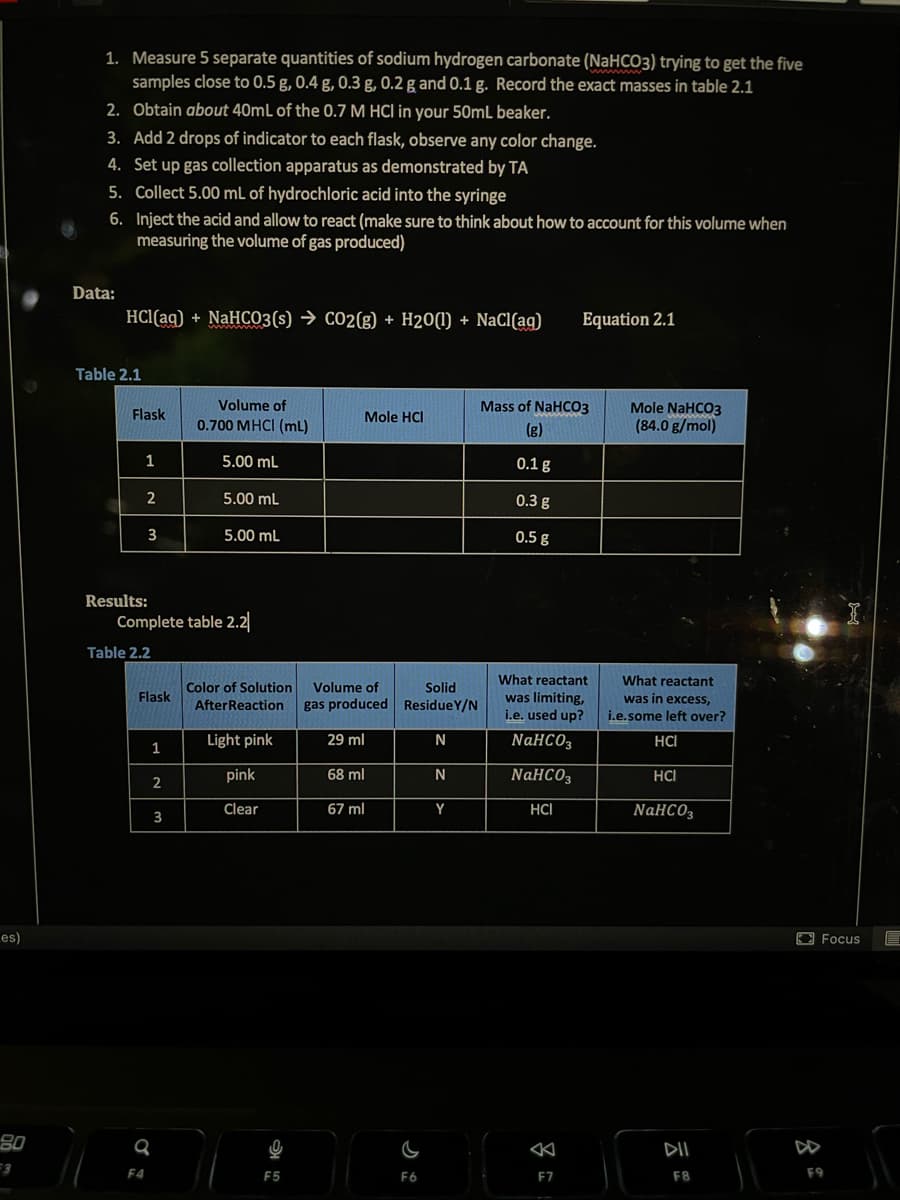
1. Measure 5 separate quantities of sodium hydrogen carbonate (NaHCO3) trying to get the five
samples close to 0.5 g, 0.4 g, 0.3 g, 0.2 g and 0.1 g. Record the exact masses in table 2.1
2. Obtain about 40mL of the 0.7 M HCI in your 50mL beaker.
3. Add 2 drops of indicator to each flask, observe any color change.
4. Set up gas collection apparatus as demonstrated by TA
5. Collect 5.00 mL of hydrochloric acid into the syringe
6.
Inject the acid and allow to react (make sure to think about how to account for this volume when
measuring the volume of gas produced)
Data:
HCl(aq) + NaHCO3(s) → CO2(g) + H20(1) + NaCl(aq)
Table 2.1
Flask
1
2
3
Results:
Table 2.2
F4
Complete table 2.2
Flask
1
2
Q
Volume of
0.700 MHCI (ml)
5.00 mL
5.00 mL
3
5.00 mL
Color of Solution
After Reaction
Light pink
pink
Clear
F5
Mole HCI
Volume of
gas produced
29 ml
68 ml
67 ml
Solid
ResidueY/N
F6
N
N
Y
Equation 2.1
Mass of NaHCO3
(g)
0.1 g
0.3 g
0.5 g
What reactant
was limiting,
i.e. used up?
NaHCO3
NaHCO3
HCI
F7
Mole NaHCO3
(84.0 g/mol)
What reactant
was in excess,
i.e.some left over?
HCI
HCI
NaHCO3
DII
F8
Focus
F9
Transcribed Image Text:Analysis:
1. Reconcile your prediction with your observations. Write a definition for limiting and excess
reactants based on your observations.
2. Calculate the number of moles of the excess reactant that is left over in each of the reaction trials:
Trial 1:
Trial 2:
Trial 3:
I
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax