Answer the following questions using data below the questions (all under standard conditions). a Is H2 (9) capable of reducing Ni²+(ag)? Assume that the reaction occurs at standard electrochemical conditions. Half-Reaction E° (V) 2H* + 2e + H2 Ni2+ + 2e Ni -0.23 0.00 yes no b Will Sn+ oxidize Fe2+ to Fe+? Assume that the reaction occurs at standard electrochemical conditions. Half-Reaction E (V) Sn+ + 2e + Sn2+ 0.15 Fe+ +e Fe²+ 0.77 yes no C Is Fe+ (g) capable of reducing VO2+(ag)? Assume that the reaction occurs at standard electrochemical conditions. Half-Reaction E (V) Fe+ +e → Fe+ VO,+ + 2H+ + e + Vo2+ + H2O 0.77 1.00 yes no
Answer the following questions using data below the questions (all under standard conditions). a Is H2 (9) capable of reducing Ni²+(ag)? Assume that the reaction occurs at standard electrochemical conditions. Half-Reaction E° (V) 2H* + 2e + H2 Ni2+ + 2e Ni -0.23 0.00 yes no b Will Sn+ oxidize Fe2+ to Fe+? Assume that the reaction occurs at standard electrochemical conditions. Half-Reaction E (V) Sn+ + 2e + Sn2+ 0.15 Fe+ +e Fe²+ 0.77 yes no C Is Fe+ (g) capable of reducing VO2+(ag)? Assume that the reaction occurs at standard electrochemical conditions. Half-Reaction E (V) Fe+ +e → Fe+ VO,+ + 2H+ + e + Vo2+ + H2O 0.77 1.00 yes no
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter20: Chemistry Of Selected Transition Elements And Coordination Compounds
Section: Chapter Questions
Problem 107QRT:
Repeat the directions for Question 106 using a cell constructed of a strip of nickel immersed in a...
Related questions
Question
100%
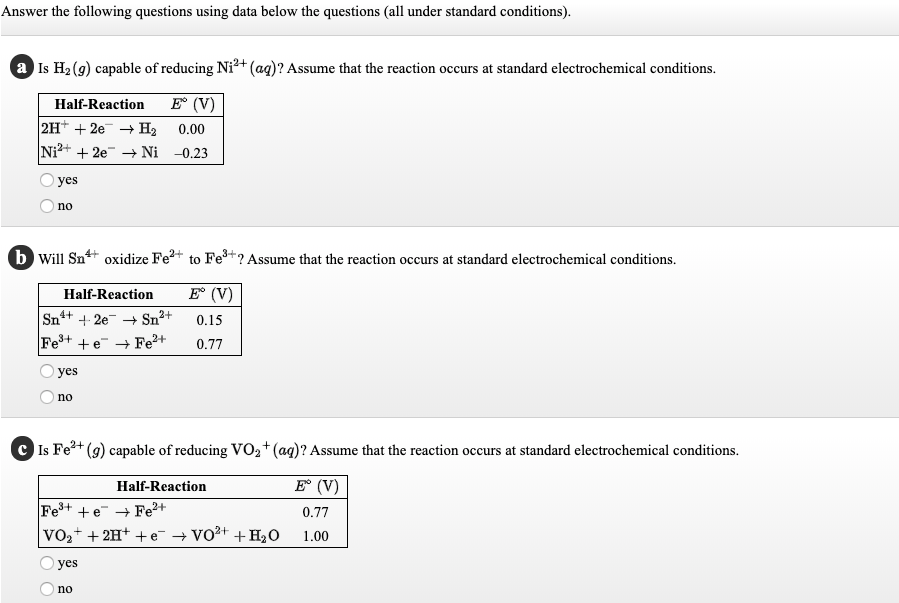
Transcribed Image Text:Answer the following questions using data below the questions (all under standard conditions).
a Is H2 (9) capable of reducing Ni²+(ag)? Assume that the reaction occurs at standard electrochemical conditions.
Half-Reaction
E° (V)
2H* + 2e + H2
Ni2+ + 2e Ni -0.23
0.00
yes
no
b Will Sn+ oxidize Fe2+ to Fe+? Assume that the reaction occurs at standard electrochemical conditions.
Half-Reaction
E (V)
Sn+ + 2e + Sn2+
0.15
Fe+ +e Fe²+
0.77
yes
no
C Is Fe+
(g) capable of reducing VO2+(ag)? Assume that the reaction occurs at standard electrochemical conditions.
Half-Reaction
E (V)
Fe+ +e → Fe+
VO,+ + 2H+ + e + Vo2+ + H2O
0.77
1.00
yes
no
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning