Anthracene is a yellow, crystalline solid found in coal tar Complete the structure for anthracene, C14 H0, by adding bonds and hydrogen atoms as necessary. Rings More Select Draw Erase C H е What type of hybrid orbitals are utilized by carbon in anthracene? How many o bonds and t bonds are there in an anthracene molecule? o bonds: bonds How many valence electrons occupy o-bond orbitals, and how many occupy T-bond orbitals? valence e in g-bond orbitals: valence e in T-bond orbitals:
Anthracene is a yellow, crystalline solid found in coal tar Complete the structure for anthracene, C14 H0, by adding bonds and hydrogen atoms as necessary. Rings More Select Draw Erase C H е What type of hybrid orbitals are utilized by carbon in anthracene? How many o bonds and t bonds are there in an anthracene molecule? o bonds: bonds How many valence electrons occupy o-bond orbitals, and how many occupy T-bond orbitals? valence e in g-bond orbitals: valence e in T-bond orbitals:
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter9: Bonding And Molecular Structure: Orbital Hybridization And Molecular Orbitals
Section: Chapter Questions
Problem 35GQ: Numerous molecules are detected in deep space. Three of them are illustrated here. (a) Are these...
Related questions
Question
100%
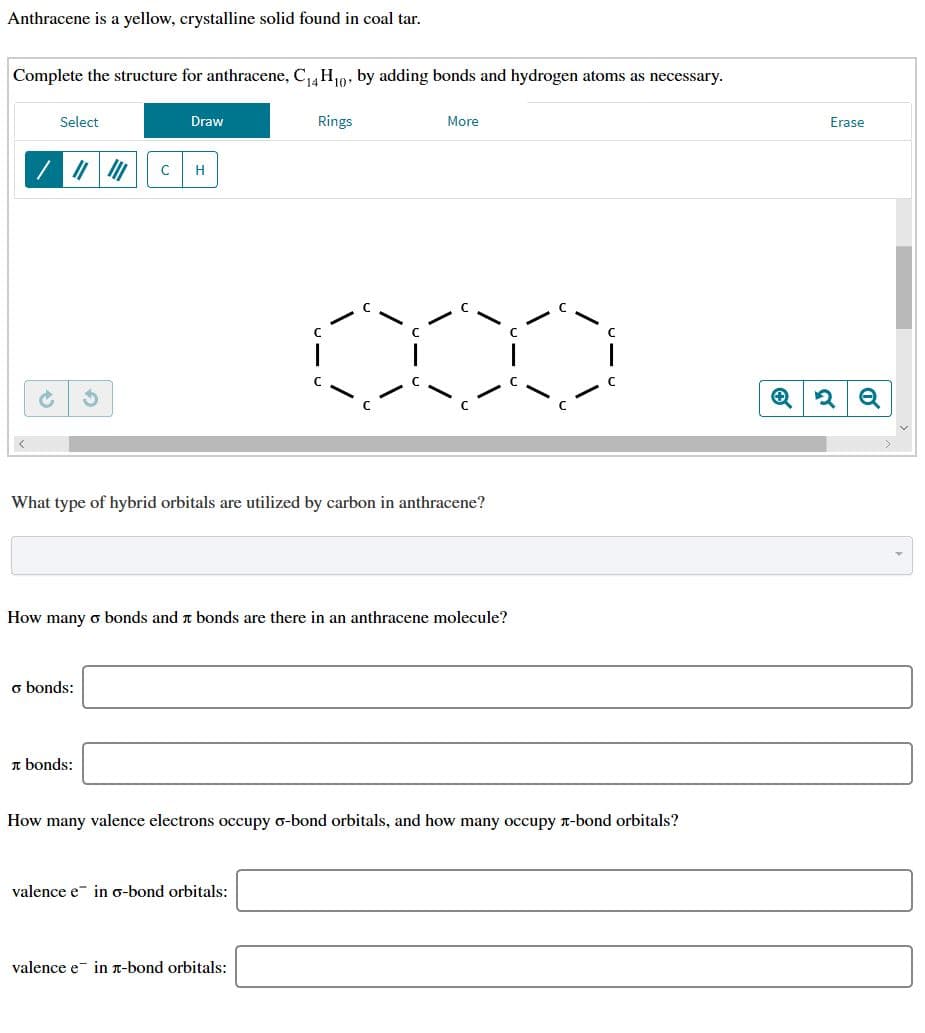
Transcribed Image Text:Anthracene is a yellow, crystalline solid found in coal tar
Complete the structure for anthracene, C14 H0, by adding bonds and hydrogen atoms as necessary.
Rings
More
Select
Draw
Erase
C
H
е
What type of hybrid orbitals are utilized by carbon in anthracene?
How many o bonds and t bonds are there in an anthracene molecule?
o bonds:
bonds
How many valence electrons occupy o-bond orbitals, and how many occupy T-bond orbitals?
valence e
in g-bond orbitals:
valence e
in T-bond orbitals:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax