"Application of the theory of the crystalline field" and your knowledge theory of the crystalline field, answer the following questions: a) Given the following graph, explain why VF1 and NiF2 have higher gr. energy crystalline with respect to the expected trend for the divale halides of the metals from the first transition series. Justify your answe: experimental line Zn?- . -- - expected line Mn2. Ca MF, of first row transition metals do d' d? d d d d6 d? ds d° d!o Lattice Energy
"Application of the theory of the crystalline field" and your knowledge theory of the crystalline field, answer the following questions: a) Given the following graph, explain why VF1 and NiF2 have higher gr. energy crystalline with respect to the expected trend for the divale halides of the metals from the first transition series. Justify your answe: experimental line Zn?- . -- - expected line Mn2. Ca MF, of first row transition metals do d' d? d d d d6 d? ds d° d!o Lattice Energy
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter21: The Solid State: Crystals
Section: Chapter Questions
Problem 21.32E: Consider Figure 21.21. If the lower rightmost corner of the unit cell were selected arbitrarily as...
Related questions
Question
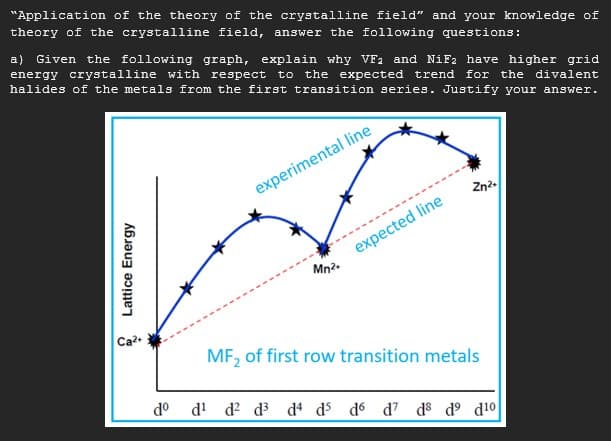
Transcribed Image Text:"Application of the theory of the crystalline field" and your knowledge of
theory of the crystalline field, answer the following questions:
a) Given the following graph, explain why VF2 and NiF2 have higher grid
energy crystalline with respect to the expected trend for the divalent
halides of the metals from the first transition series. Justify your answer.
experimental line
Zn2.
-------------------.
expected line
Mn2.
Ca2
MF, of first row transition metals
do d! d? d³ d d5 do d7 d® d° d!o
* Lattice Eners
nergy
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,