Mn, Fe, and Co in the +2 and +3 oxidation states all form hexaaquacomplexes in acidic aqueous solution. The reduction reactions of the three species are represented schematically below, where the water ligands are not shown for simplicity. It is an experimental fact from elec- trochemistry that Mn2+ and Co²+ are more easily reduced than Fe3+; that is, they will more readily accept an elec- tron. Based on the electron configurations of the ions involved, explain why Fe³+ is harder to reduce than Mn2+ and Co²+. Mn3+ + e – Mn²+ Fe* + e –→ Fe²+ Co³+ + e → Co²+
Mn, Fe, and Co in the +2 and +3 oxidation states all form hexaaquacomplexes in acidic aqueous solution. The reduction reactions of the three species are represented schematically below, where the water ligands are not shown for simplicity. It is an experimental fact from elec- trochemistry that Mn2+ and Co²+ are more easily reduced than Fe3+; that is, they will more readily accept an elec- tron. Based on the electron configurations of the ions involved, explain why Fe³+ is harder to reduce than Mn2+ and Co²+. Mn3+ + e – Mn²+ Fe* + e –→ Fe²+ Co³+ + e → Co²+
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter8: Bonding In Transition Metal Compounds And Coordination Complexes
Section: Chapter Questions
Problem 34P
Related questions
Question
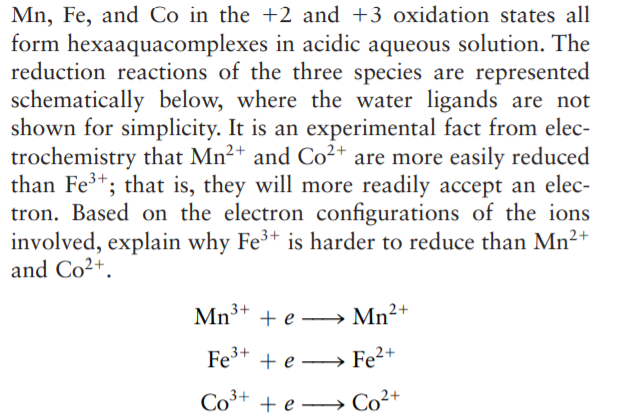
Transcribed Image Text:Mn, Fe, and Co in the +2 and +3 oxidation states all
form hexaaquacomplexes in acidic aqueous solution. The
reduction reactions of the three species are represented
schematically below, where the water ligands are not
shown for simplicity. It is an experimental fact from elec-
trochemistry that Mn2+ and Co²+ are more easily reduced
than Fe3+; that is, they will more readily accept an elec-
tron. Based on the electron configurations of the ions
involved, explain why Fe³+ is harder to reduce than Mn2+
and Co²+.
Mn3+ + e – Mn²+
Fe* + e –→ Fe²+
Co³+
+ e → Co²+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning