As your undergraduate thesis, you developed a colorimetric method for determining a certain metal (M) found in your local water supply. You achieved this by reacting the metal with an organic ligand that produced a colored complex that strongly absorbs the wavelength at 478 nm. The following results were obtained for standard solutions of nitrite and for a sample containing an unknown amount: mg/L metal Absorbance 0.000 0.000 0.100 0.052 0.200 0.110 0.400 0.214 0.500 0.281 1.000 0.556 1.500 0.816 2.000 1.078 Sample 0.114 d. Assuming the path length of your beam is 1.00 cm, which is the common size for a cuvette, what is the absorptivity of your solution? e. What is the concentration (mg/L) of the metal in your sample? Show your calculation, follow format.
As your undergraduate thesis, you developed a colorimetric method for determining a certain metal (M) found in your local water supply. You achieved this by reacting the metal with an organic ligand that produced a colored complex that strongly absorbs the wavelength at 478 nm. The following results were obtained for standard solutions of nitrite and for a sample containing an unknown amount: mg/L metal Absorbance 0.000 0.000 0.100 0.052 0.200 0.110 0.400 0.214 0.500 0.281 1.000 0.556 1.500 0.816 2.000 1.078 Sample 0.114 d. Assuming the path length of your beam is 1.00 cm, which is the common size for a cuvette, what is the absorptivity of your solution? e. What is the concentration (mg/L) of the metal in your sample? Show your calculation, follow format.
Chapter24: Introduction To Spectrochemical Methods
Section: Chapter Questions
Problem 24.21QAP
Related questions
Question
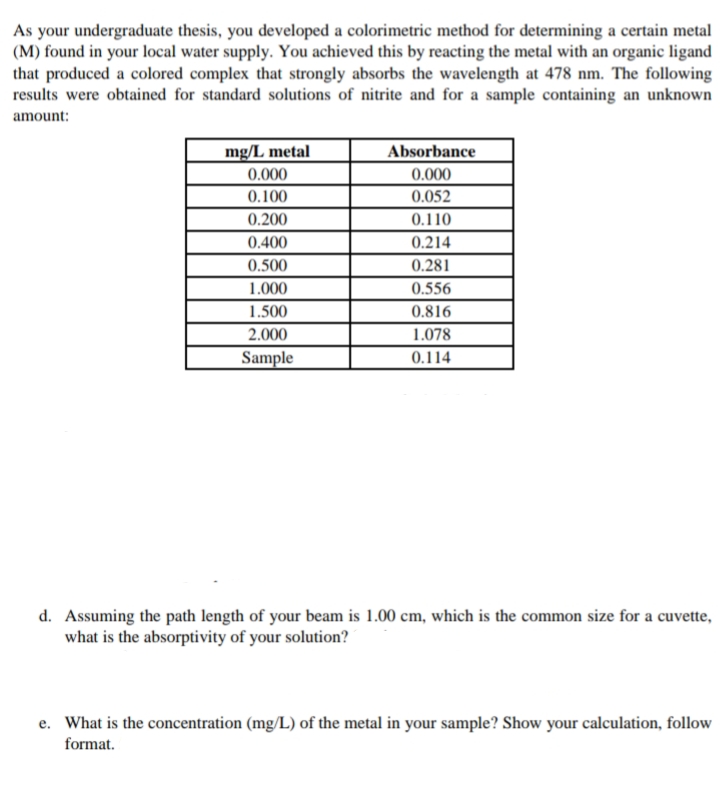
Transcribed Image Text:As your undergraduate thesis, you developed a colorimetric method for determining a certain metal
(M) found in your local water supply. You achieved this by reacting the metal with an organic ligand
that produced a colored complex that strongly absorbs the wavelength at 478 nm. The following
results were obtained for standard solutions of nitrite and for a sample containing an unknown
amount:
mg/L metal
Absorbance
0.000
0.000
0.100
0.052
0.200
0.110
0.400
0.214
0.500
0.281
1.000
0.556
1.500
0.816
2.000
1.078
Sample
0.114
d. Assuming the path length of your beam is 1.00 cm, which is the common size for a cuvette,
what is the absorptivity of your solution?
e. What is the concentration (mg/L) of the metal in your sample? Show your calculation, follow
format.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning