At 37 °C, the serine protease subtilisin has kat = 50 s1 and KM= 1.4 x 10-4 M. It is proposed that the N155 side chain contributes a hydrogen bond to the oxyanion hole of subtilisin. J. A. Wells and col- leagues reported (1986, Phil. Trans. R. Soc. Lond. A 317:415–423) the following kinetic parameters for the N155T mutant of subtilisin: kat = 0.02 s-' and KM = 2 × 10-4 M. (a) Subtilisin is used in some laundry detergents to help remove protein-type stains. What unusual kind of stability does this suggest for subtilisin? (b) Subtilisin does have a problem, in that it becomes inactivated by oxidation of a methionine close to the active site. Suggest a way to make a better subtilisin. (c) Is the effect of the N155T mutation what you would expect for a residue that makes up part of the oxyanion hole? How do the reported values of kat and KM support your answer? (d) Assuming that the T155 side chain cannot H-bond to the oxyanion intermediate, by how much (in kJ/mol) does N155 appear to stabilize the transition state at 37 °C? (e) The value you calculated in part (d) represents the strength of the H-bond between N155 and the oxyanion in the transition state. This value is higher than typical H-bonds in water. How might this observation be rationalized? Hint: consider Equation 2.2 (Coulomb's Law).
At 37 °C, the serine protease subtilisin has kat = 50 s1 and KM= 1.4 x 10-4 M. It is proposed that the N155 side chain contributes a hydrogen bond to the oxyanion hole of subtilisin. J. A. Wells and col- leagues reported (1986, Phil. Trans. R. Soc. Lond. A 317:415–423) the following kinetic parameters for the N155T mutant of subtilisin: kat = 0.02 s-' and KM = 2 × 10-4 M. (a) Subtilisin is used in some laundry detergents to help remove protein-type stains. What unusual kind of stability does this suggest for subtilisin? (b) Subtilisin does have a problem, in that it becomes inactivated by oxidation of a methionine close to the active site. Suggest a way to make a better subtilisin. (c) Is the effect of the N155T mutation what you would expect for a residue that makes up part of the oxyanion hole? How do the reported values of kat and KM support your answer? (d) Assuming that the T155 side chain cannot H-bond to the oxyanion intermediate, by how much (in kJ/mol) does N155 appear to stabilize the transition state at 37 °C? (e) The value you calculated in part (d) represents the strength of the H-bond between N155 and the oxyanion in the transition state. This value is higher than typical H-bonds in water. How might this observation be rationalized? Hint: consider Equation 2.2 (Coulomb's Law).
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter29: Supercritical Fluid Chromatography And Extraction
Section: Chapter Questions
Problem 29.11QAP
Related questions
Question
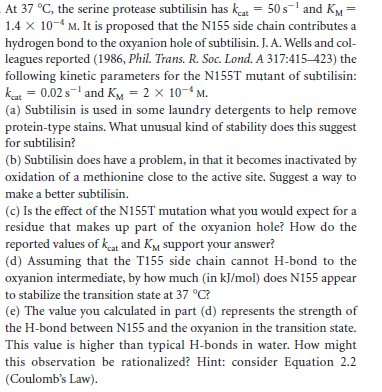
Transcribed Image Text:At 37 °C, the serine protease subtilisin has kat = 50 s1 and KM=
1.4 x 10-4 M. It is proposed that the N155 side chain contributes a
hydrogen bond to the oxyanion hole of subtilisin. J. A. Wells and col-
leagues reported (1986, Phil. Trans. R. Soc. Lond. A 317:415–423) the
following kinetic parameters for the N155T mutant of subtilisin:
kat = 0.02 s-' and KM = 2 × 10-4 M.
(a) Subtilisin is used in some laundry detergents to help remove
protein-type stains. What unusual kind of stability does this suggest
for subtilisin?
(b) Subtilisin does have a problem, in that it becomes inactivated by
oxidation of a methionine close to the active site. Suggest a way to
make a better subtilisin.
(c) Is the effect of the N155T mutation what you would expect for a
residue that makes up part of the oxyanion hole? How do the
reported values of kat and KM support your answer?
(d) Assuming that the T155 side chain cannot H-bond to the
oxyanion intermediate, by how much (in kJ/mol) does N155 appear
to stabilize the transition state at 37 °C?
(e) The value you calculated in part (d) represents the strength of
the H-bond between N155 and the oxyanion in the transition state.
This value is higher than typical H-bonds in water. How might
this observation be rationalized? Hint: consider Equation 2.2
(Coulomb's Law).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning