Atoms vibrate relative to one another in molecules with the bond acting as a spring. Consider the H – CI bond, where the heavy Cl atom forms a stationary anchor for the very light H atom. That is, only the H atom moves, vibrating as a simple harmonic oscillator. (a) Give the equation that describes the allowed vibrational energy levels of the bond. (b) The force constant kf for the H – Cl bond is 516.3 N m'. Given the mass of H equal to 1.7 x 1027 kg, determine the difference in energy (separation) between adjacent energy levels. (c) Calculate the zero-point energy of this molecular oscillator.
Atoms vibrate relative to one another in molecules with the bond acting as a spring. Consider the H – CI bond, where the heavy Cl atom forms a stationary anchor for the very light H atom. That is, only the H atom moves, vibrating as a simple harmonic oscillator. (a) Give the equation that describes the allowed vibrational energy levels of the bond. (b) The force constant kf for the H – Cl bond is 516.3 N m'. Given the mass of H equal to 1.7 x 1027 kg, determine the difference in energy (separation) between adjacent energy levels. (c) Calculate the zero-point energy of this molecular oscillator.
Modern Physics
3rd Edition
ISBN:9781111794378
Author:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Chapter11: Molecular Structure
Section: Chapter Questions
Problem 15P
Related questions
Question
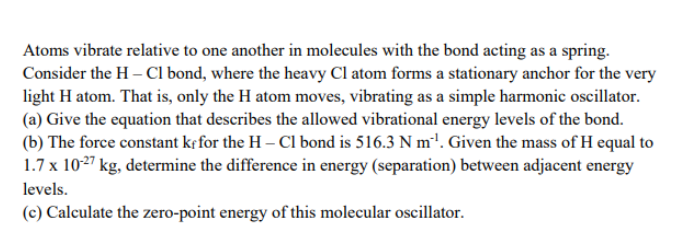
Transcribed Image Text:Atoms vibrate relative to one another in molecules with the bond acting as a spring.
Consider the H – CI bond, where the heavy Cl atom forms a stationary anchor for the very
light H atom. That is, only the H atom moves, vibrating as a simple harmonic oscillator.
(a) Give the equation that describes the allowed vibrational energy levels of the bond.
(b) The force constant kf for the H – Cl bond is 516.3 N m'1. Given the mass of H equal to
1.7 x 1027 kg, determine the difference in energy (separation) between adjacent energy
levels.
(c) Calculate the zero-point energy of this molecular oscillator.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Modern Physics
Physics
ISBN:
9781111794378
Author:
Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:
Cengage Learning

University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Modern Physics
Physics
ISBN:
9781111794378
Author:
Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:
Cengage Learning

University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning