The atomic radii of X+n and Y-m ions are 0.072and 0.133 nm, respectively. Calculate the attractive energy between these two ions at their equilibrium interionic separation. n= 4 y=3
The atomic radii of X+n and Y-m ions are 0.072and 0.133 nm, respectively. Calculate the attractive energy between these two ions at their equilibrium interionic separation. n= 4 y=3
Modern Physics
3rd Edition
ISBN:9781111794378
Author:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Chapter12: The Solid State
Section: Chapter Questions
Problem 7P
Related questions
Question
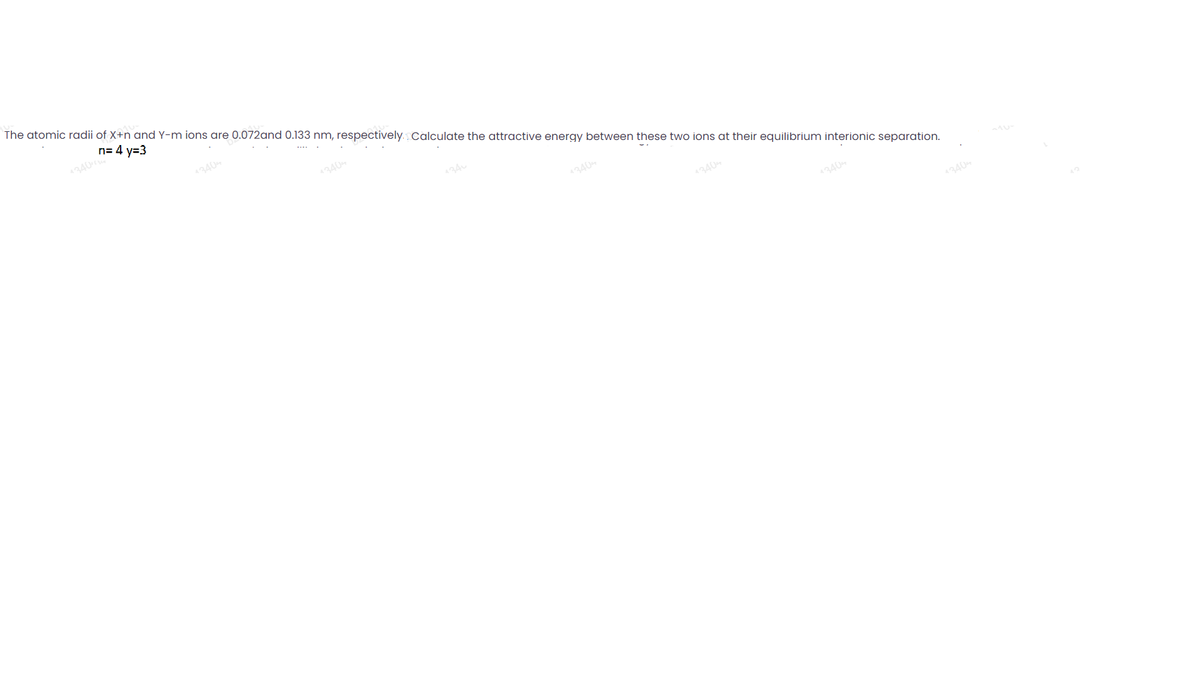
Transcribed Image Text:The atomic radii of X+n and Y-m ions are 0.072and 0.133 nm, respectively. Calculate the attractive energy between these two ions at their equilibrium interionic separation.
n= 4 y=3
340
1340
240
1340
1340
340
1340
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Modern Physics
Physics
ISBN:
9781111794378
Author:
Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:
Cengage Learning

Classical Dynamics of Particles and Systems
Physics
ISBN:
9780534408961
Author:
Stephen T. Thornton, Jerry B. Marion
Publisher:
Cengage Learning

University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax

Modern Physics
Physics
ISBN:
9781111794378
Author:
Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:
Cengage Learning

Classical Dynamics of Particles and Systems
Physics
ISBN:
9780534408961
Author:
Stephen T. Thornton, Jerry B. Marion
Publisher:
Cengage Learning

University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning