B. Classification of Bonding based on Electronegativity Complete the table below based on what is asked. More electronegative Less electronegative element and value Bonding Difference in electronegativity Bond Type between element and value Sulfur and Hydrogen Sulfur and Sulfur - 2.5 Hydrogen - 2.1 0.4 Nonpolar covalent bond cesium Chlorine and bromine Calcium and chlorine Oxygen and hydrogen Nitrogen and hydrogen
B. Classification of Bonding based on Electronegativity Complete the table below based on what is asked. More electronegative Less electronegative element and value Bonding Difference in electronegativity Bond Type between element and value Sulfur and Hydrogen Sulfur and Sulfur - 2.5 Hydrogen - 2.1 0.4 Nonpolar covalent bond cesium Chlorine and bromine Calcium and chlorine Oxygen and hydrogen Nitrogen and hydrogen
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter2: Lewis Structures
Section: Chapter Questions
Problem 6CTQ: It is impossible to draw a legitimate Lewis structure of a neutral NH4 molecule. Hypothetically,how...
Related questions
Question
Transcribed Image Text:2.1
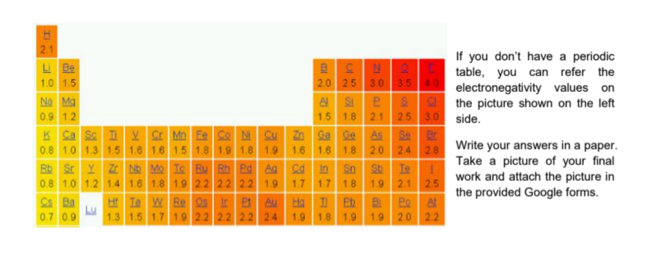
If you don't have a periodic
table, you can refer the
electronegativity values on
the picture shown on the left
3.0
L Be
1.0 1.5
2.0 25 3.0 3.540
No Ma
0.9 1.2
1.5 1.8
2.1 25
side.
K Ca se T
Zn Ga se
1.6 1.8
V Mn Ee Ce N C
0.8 1.0 1.3 1.5 1.6 1.6 15 1.8 1.9 1.8 1.9
Rb Sr Y Z N Mo Ie Bu Rh Pd A
0.8 1.0 1.2 1.4 1.6 1.8 1.9 22 22 2.2 1.9
Ht Ia w Re s Ir e Au
As
Se
Write your answers in a paper.
Take a picture of your final
work and attach the picture in
1.6
2.0
2.4 28
in
Ie
1.7
1.7
1.8
1.9
2.1
25
the provided Google forms.
Ba
0.7 0.9
Eb
1.3 1.5 1.7 1.9 22 22 2.2 24 1.9 1.8 1.9
Ha
1.9
2.0 22
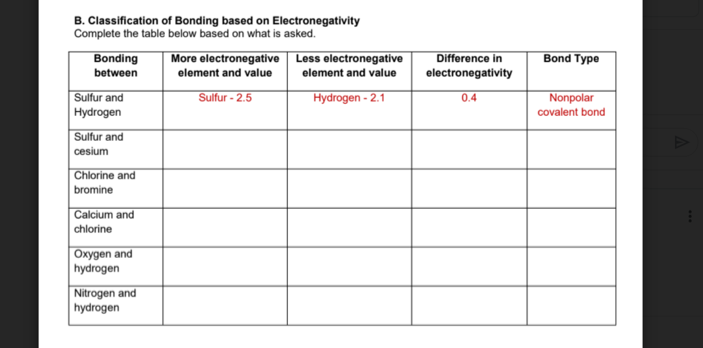
Transcribed Image Text:B. Classification of Bonding based on Electronegativity
Complete the table below based on what is asked.
More electronegative | Less electronegative
element and value
Bonding
Difference in
Bond Type
between
element and value
electronegativity
Sulfur - 2.5
Hydrogen - 2.1
0.4
Nonpolar
Sulfur and
Hydrogen
covalent bond
Sulfur and
cesium
Chlorine and
bromine
Calcium and
chlorine
Oxygen and
hydrogen
Nitrogen and
hydrogen
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning