B. Determine the following: Pm, Pv, Pm/v, XA, m, and M if 107.125 grams NaOH is dissolved in 480 ml of water; if the density of NaOH is 2.13 g/mL and density of H20 is 1.00 g/mL. (Answers should be 2 decimal places) MASS DENSITY MOLAR MASS VOLUME MOLE |2.13 g/ml SOLUTE 107.125 50.30 mL grams NaOH SOLVENT 480 grams of water 480 mL of 1.00 g/mL. water SOLUTION 587.13 3.13 g/mL 530.3 mL solution
B. Determine the following: Pm, Pv, Pm/v, XA, m, and M if 107.125 grams NaOH is dissolved in 480 ml of water; if the density of NaOH is 2.13 g/mL and density of H20 is 1.00 g/mL. (Answers should be 2 decimal places) MASS DENSITY MOLAR MASS VOLUME MOLE |2.13 g/ml SOLUTE 107.125 50.30 mL grams NaOH SOLVENT 480 grams of water 480 mL of 1.00 g/mL. water SOLUTION 587.13 3.13 g/mL 530.3 mL solution
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.16QAP
Related questions
Question
finish the table
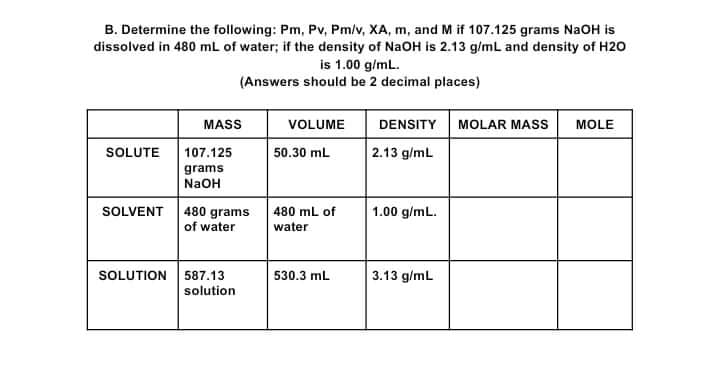
Transcribed Image Text:B. Determine the following: Pm, Pv, Pm/v, XA, m, and M if 107.125 grams NaOH is
dissolved in 480 ml of water; if the density of NaOH is 2.13 g/mL and density of H20
is 1.00 g/mL.
(Answers should be 2 decimal places)
MASS
DENSITY MOLAR MASS
VOLUME
MOLE
2.13 g/ml
SOLUTE
107.125
50.30 mL
grams
NaOH
SOLVENT 480 grams
of water
480 mL of
1.00 g/mL.
water
SOLUTION 587.13
solution
3.13 g/mL
530.3 mL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

