B3 Initial water temperature 21 °C B4 Final water temperature 100 °C B5 Temperature change (^ T) 100-21=79 °c B6 Volume of water Mass of water B7 Number of calories needed to boil water (show your calculation)
B3 Initial water temperature 21 °C B4 Final water temperature 100 °C B5 Temperature change (^ T) 100-21=79 °c B6 Volume of water Mass of water B7 Number of calories needed to boil water (show your calculation)
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter14: Liquids And Solids
Section: Chapter Questions
Problem 9ALQ: ook at Fig. 14.2. Why doesn't temperature increase continuously ever time? That is, why does the...
Related questions
Question
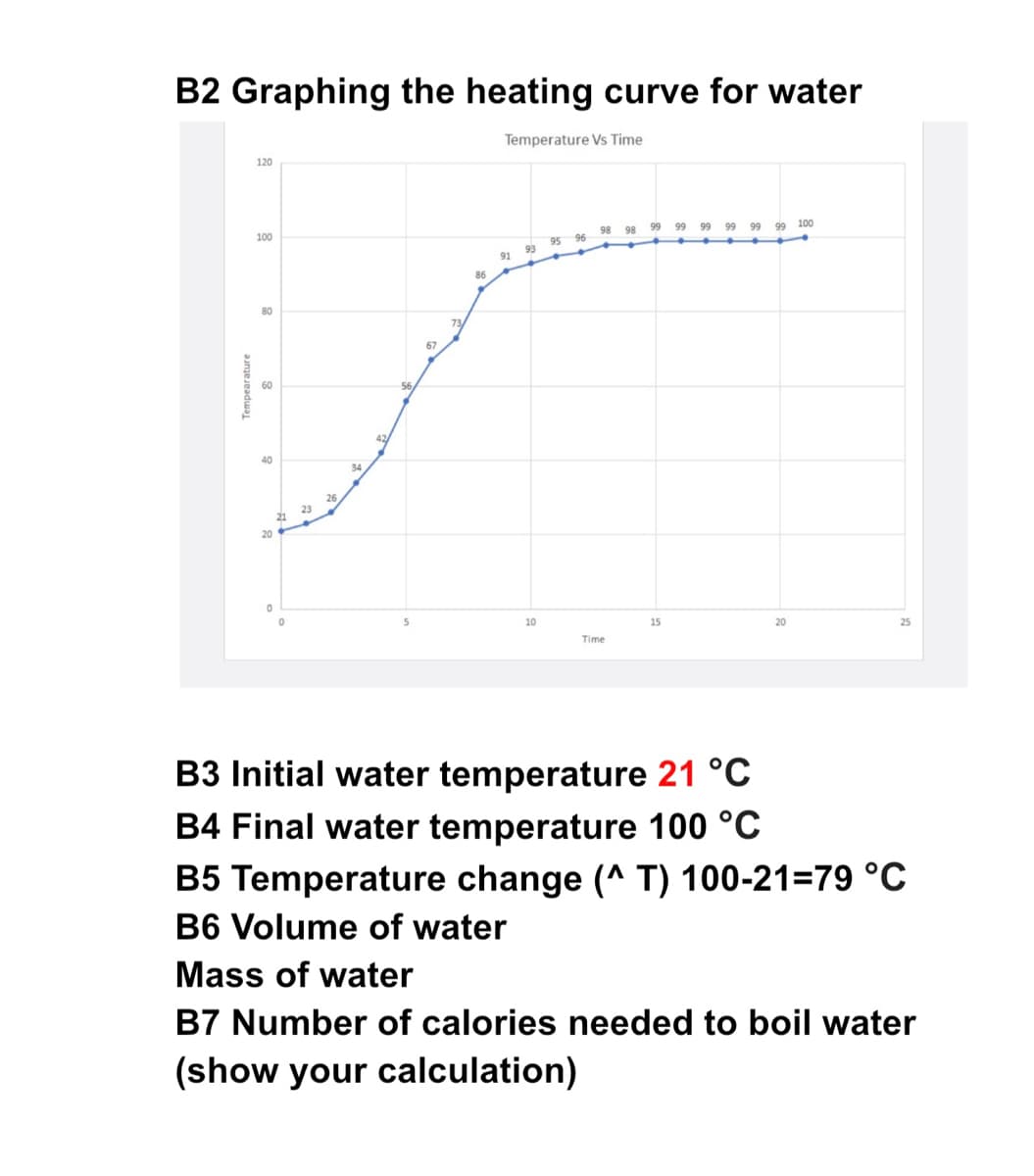
Transcribed Image Text:B2 Graphing the heating curve for water
Temperature Vs Time
120
100
98
98 99
99 99
99
99
99 100
96
95
93
91
86
80
73
67
56,
40
34
26
23
21
20
5
10
15
20
25
Time
B3 Initial water temperature 21 °C
B4 Final water temperature 100 °C
B5 Temperature change (^ T) 100-21=79 °C
B6 Volume of water
Mass of water
B7 Number of calories needed to boil water
(show your calculation)
Tempearature
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning