Mass of iron 64.8973 g Mass of water 61.9272 g Initial temperature of iron (boiling water Tm) 98.0 °C Initial temperature of calorimeter + water, T; 22.1 °C Final temperature, T¡ 30.3 °C
Mass of iron 64.8973 g Mass of water 61.9272 g Initial temperature of iron (boiling water Tm) 98.0 °C Initial temperature of calorimeter + water, T; 22.1 °C Final temperature, T¡ 30.3 °C
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter12: Thermodynamic Processes And Thermochemistry
Section: Chapter Questions
Problem 14P
Related questions
Question
Please how I find the q Fe,q water, q cal with the ungrounded and rounded values please.
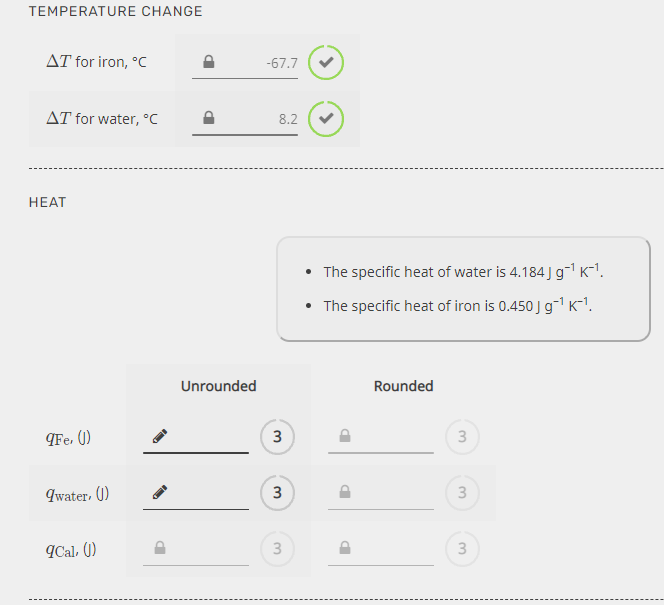
Transcribed Image Text:TEMPERATURE CHANGE
AT for iron, °C
-67.7
AT for water, °C
8.2
НЕАТ
• The specific heat of water is 4.184 Jg K-!.
• The specific heat of iron is 0.450 J g-1 K-1.
Unrounded
Rounded
(Fe. ()
Iwater, ()
3
Cal, ()
3
3.
3.
3.

Transcribed Image Text:DATA
Mass of iron
64.8973 g
Mass of water
61.9272 g
Initial temperature of iron (boiling water Tm)
98.0 °C
Initial temperature of calorimeter + water, T;
22.1 °C
Final temperature, Tf
30.3 °C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,