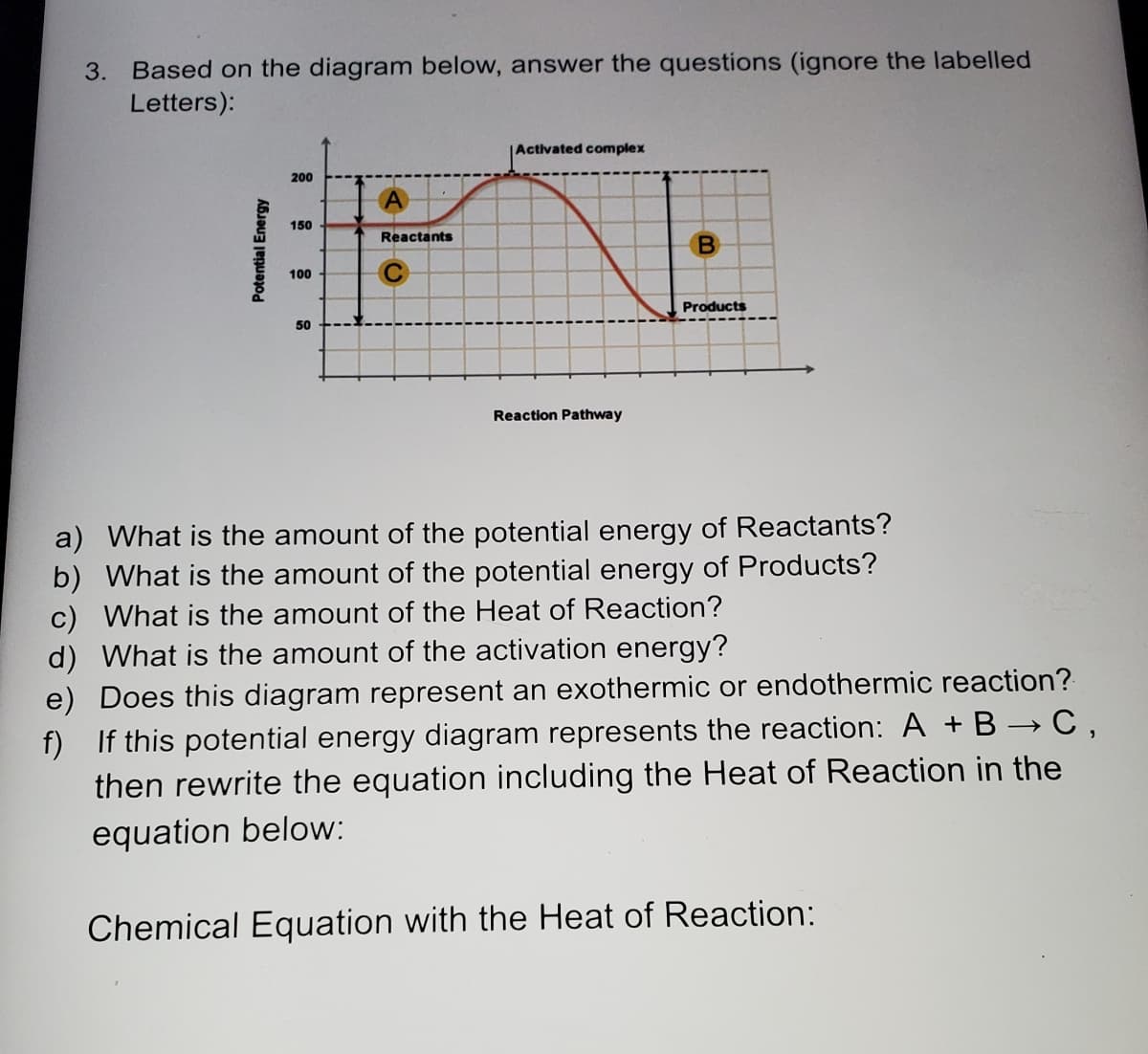
Based on the diagram below, answer the questions (ignore the labelled Letters): Activated complex 200 150 Reactants 100 Products 50 Reaction Pathway Vhat is the amount of the potential energy of Reactants? Vhat is the amount of the potential energy of Products? What is the amount of the Heat of Reaction? /hat is the amount of the activation energy? oes this diagram represent an exothermic or endothermic reaction? this potential energy diagram represents the reaction: A +B → C, en rewrite the equation including the Heat of Reaction in the quation below: nemical Equation with the Heat of Reaction: Potential Energy
Catalysis and Enzymatic Reactions
Catalysis is the kind of chemical reaction in which the rate (speed) of a reaction is enhanced by the catalyst which is not consumed during the process of reaction and afterward it is removed when the catalyst is not used to make up the impurity in the product. The enzymatic reaction is the reaction that is catalyzed via enzymes.
Lock And Key Model
The lock-and-key model is used to describe the catalytic enzyme activity, based on the interaction between enzyme and substrate. This model considers the lock as an enzyme and the key as a substrate to explain this model. The concept of how a unique distinct key only can have the access to open a particular lock resembles how the specific substrate can only fit into the particular active site of the enzyme. This is significant in understanding the intermolecular interaction between proteins and plays a vital role in drug interaction.
Step by step
Solved in 3 steps









