Based on the previous question and table, Predict AT for the following liquids. You do not have to predict the exact value; simply rank the liquids from greatest to least AT (Greatest AT= 1 and Lowest AT = 4) Predicted AT Use intermolecular forces present for explanation (Why?) 1-butanol methanol n-pentane 2261 n-hexane Measure the unknown liquids. Identify the unknowns based on the absolute value of AT (T2-T1) and determine if your predictions above were correct. T1 (°C) T2 (°C) JAT | Compound Identity HO HO Alkane-1 24.2 _6.3 17.9 Alkane-2 27.5 16.1 26.7 14.1 12.6 Alcohol-1 Alcohol-2 29.4 15.8 13.66 29.5 20.4 9.1 Alcohol-3 29.0 20-1 8.9 Alcohol-4
Based on the previous question and table, Predict AT for the following liquids. You do not have to predict the exact value; simply rank the liquids from greatest to least AT (Greatest AT= 1 and Lowest AT = 4) Predicted AT Use intermolecular forces present for explanation (Why?) 1-butanol methanol n-pentane 2261 n-hexane Measure the unknown liquids. Identify the unknowns based on the absolute value of AT (T2-T1) and determine if your predictions above were correct. T1 (°C) T2 (°C) JAT | Compound Identity HO HO Alkane-1 24.2 _6.3 17.9 Alkane-2 27.5 16.1 26.7 14.1 12.6 Alcohol-1 Alcohol-2 29.4 15.8 13.66 29.5 20.4 9.1 Alcohol-3 29.0 20-1 8.9 Alcohol-4
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter3: Atomic Shells And Classical Models Of Chemical Bonding
Section: Chapter Questions
Problem 70P: Ozone (O3) has a nonzero dipole moment. In the molecule of O3 , one of the oxygen atoms is directly...
Related questions
Question
Transcribed Image Text:dispersio
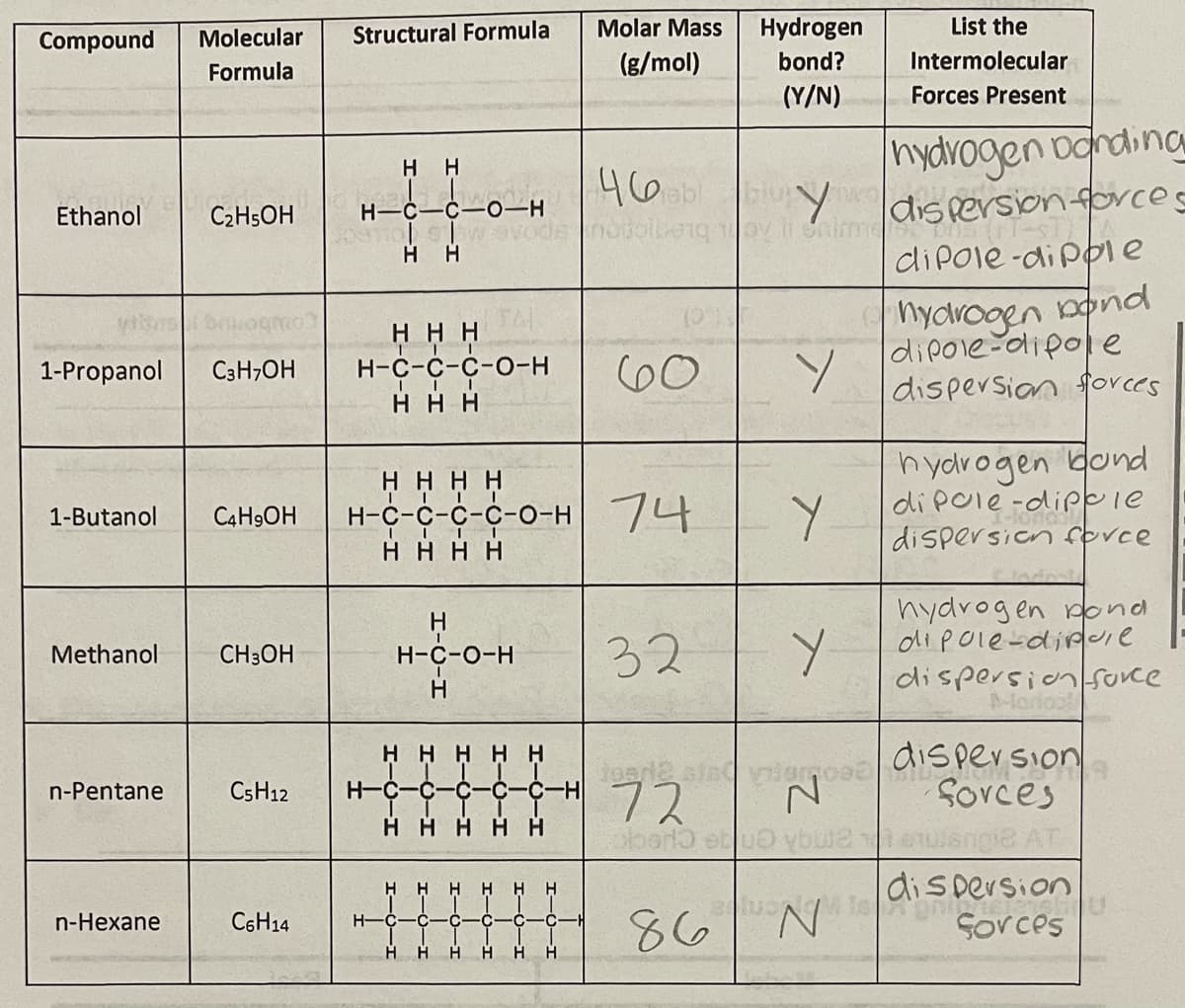
List the
Compound
Molecular
Structural Formula
Molar Mass
Hydrogen
(g/mol)
bond?
Intermolecular
Formula
(Y/N)
Forces Present
hydrogen oanding
H H
461
notolbenq oy renime
Y
dis persion forces
Ethanol
C2HSOH
H-C-C-0–H
H H
diPole-di pple
hydvogen nonod
dipole-dipole
dispersion forces
ннн
Н-с-с-с-о-н
ннн
60
1-Propanol
C3H;OH
нннн
н-с-с-с-с-о-н /4
hydrogen dond
di pole-dippie
dispersion fovce
1-Butanol
C4H9OH
нннн
hydrogen pond
dipole-dipie
dispersion Surce
H.
32
Methanol
CH3OH
H-C-O-H
Modos
H H HHH
CSH12
H-C-C
Sovces
H-
72
vaQusmue angA enge cuec
n-Pentane
H
86
N.
Sorces
n-Hexane
C6H14
-C
-C
H.
H.
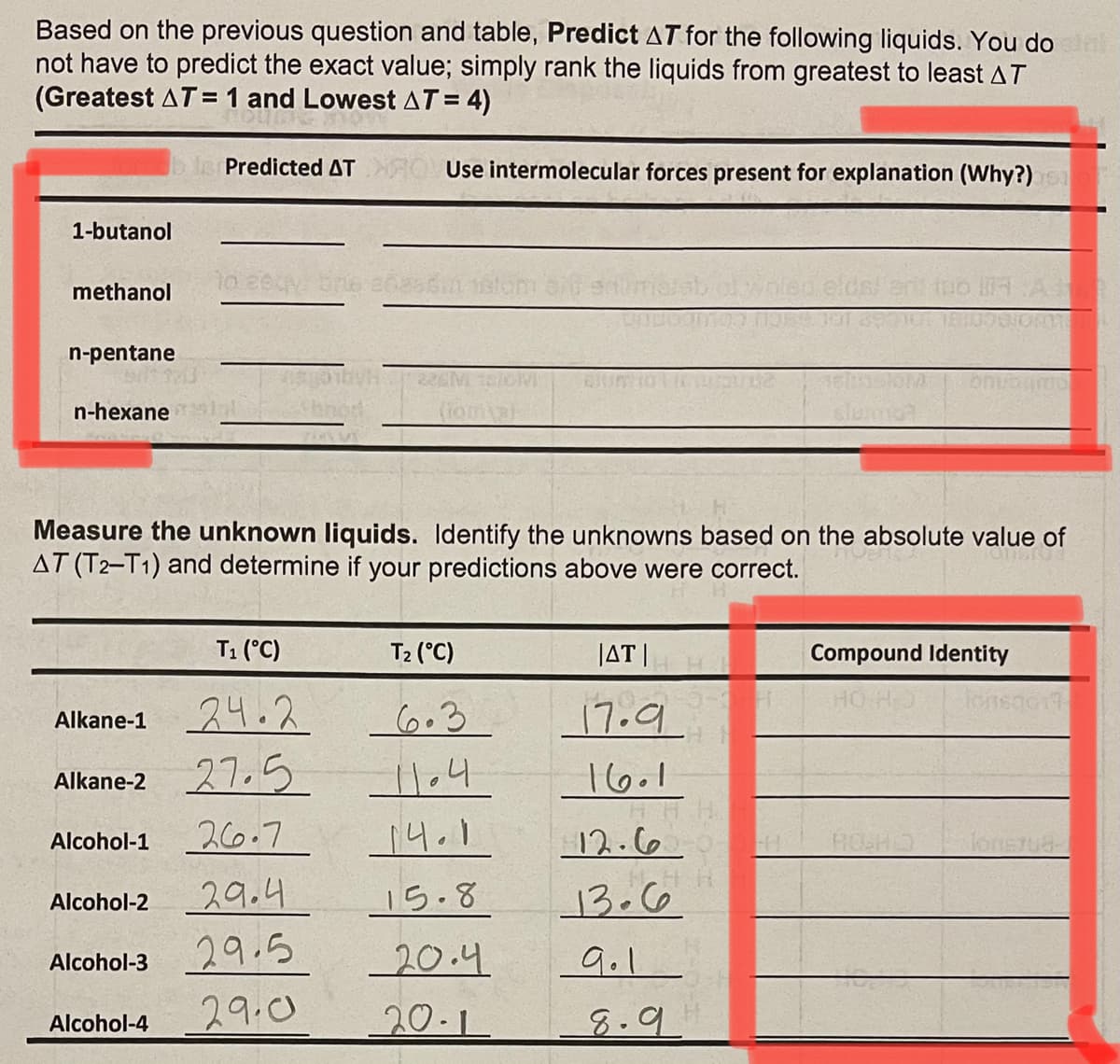
Transcribed Image Text:Based on the previous question and table, Predict AT for the following liquids. You do
not have to predict the exact value; simply rank the liquids from greatest to least AT
(Greatest AT= 1 and Lowest AT= 4)
Predicted AT
Use intermolecular forces present for explanation (Why?)
1-butanol
methanol
n-pentane
n-hexane
Measure the unknown liquids. Identify the unknowns based on the absolute value of
AT (T2-T1) and determine if your predictions above were correct.
T1 (°C)
T2 (°C)
JAT |
Compound Identity
Alkane-1 24.2
27.5
6.3
17.9.
11.4
16.1
Alkane-2
26.7
12.6
Alcohol-1
HOH
29.4
15.8
13.66
Alcohol-2
Alcohol-3 29.5
29.0
20.4
9.1
20-1
8.9
Alcohol-4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning
