Before you Begin: Use your Periodic table to complete the table: Magnesium Name Hydrogen Oxygen Carbon Socom sUlSor CI Symbol Na Atomic # Atomic Mass H.0079 15.999 # Protons # Electrons 17 Group 1 Period 1 Procedure: 1. Pick one color of bead to represent each of the elements below. Use map pencils to color the circle with the color you chose to represent the element. Hydrogen (H) Oxygen (O) Sodium (Na) le o s nodhoto mo Sulfur (S) admun woro belleo Carbon (C) Chlorine (Cl) olom rt ensom al Magnesium (Mg) a. Molecules with two atoms are linear: b. Molecules with two atoms on either side of a center atom are bent: c. Molecules with three atoms around a center atom are shaped like pyramids: 2. Given this information:
Before you Begin: Use your Periodic table to complete the table: Magnesium Name Hydrogen Oxygen Carbon Socom sUlSor CI Symbol Na Atomic # Atomic Mass H.0079 15.999 # Protons # Electrons 17 Group 1 Period 1 Procedure: 1. Pick one color of bead to represent each of the elements below. Use map pencils to color the circle with the color you chose to represent the element. Hydrogen (H) Oxygen (O) Sodium (Na) le o s nodhoto mo Sulfur (S) admun woro belleo Carbon (C) Chlorine (Cl) olom rt ensom al Magnesium (Mg) a. Molecules with two atoms are linear: b. Molecules with two atoms on either side of a center atom are bent: c. Molecules with three atoms around a center atom are shaped like pyramids: 2. Given this information:
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter9: Ionic And Covalent Bonding
Section: Chapter Questions
Problem 9.23QP
Related questions
Question
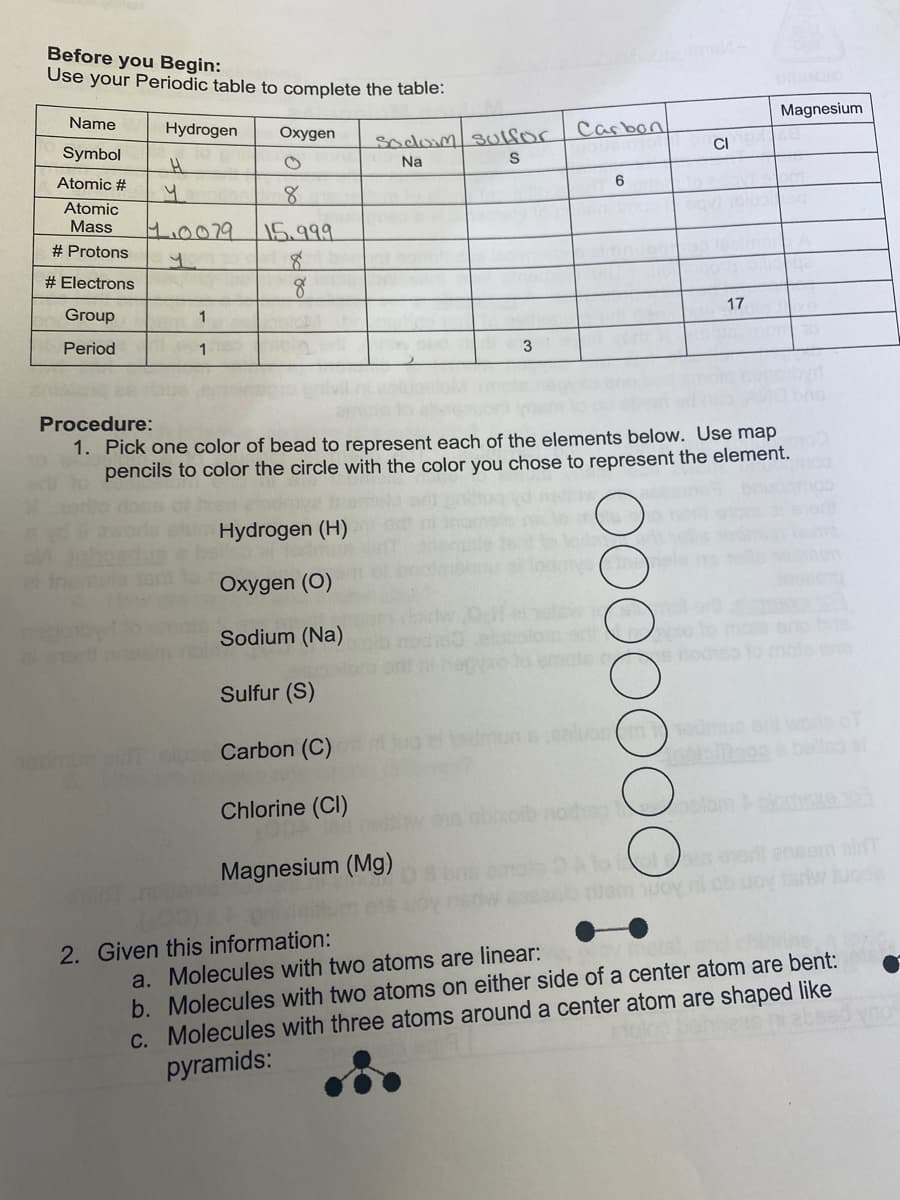
Transcribed Image Text:Before you Begin:
Use your Periodic table to complete the table:
Magnesium
Name
Hydrogen
Oxygen
Carbon
socomsUlfor
CI
Symbol
Na
Atomic #
Atomic
.0079
Mass
15.999
# Protons
# Electrons
17
Group
1
Period
1
3
Procedure:
1. Pick one color of bead to represent each of the elements below. Use map
pencils to color the circle with the color you chose to represent the element.
Hydrogen (H)
Oxygen (O)
Sodium (Na)
s nodh
to mote ono
Sulfur (S)
worlk oT
belleo a
senluomed
ouc Carbon (C)
Chlorine (CI)
ebixoib nodt
yolom
Sis eor aneem ai
w Juods
Magnesium (Mg)
a. Molecules with two atoms are linear:
b. Molecules with two atoms on either side of a center atom are bent:
c. Molecules with three atoms around a center atom are shaped like
pyramids:
2. Given this information:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning