Begining with 1 M concentrations of each reactant and product at pH=7 and 25.0 degrees C, calculate the K'eq (to one decimal point) of the reaction Pyruvate + NADH+H+ <=> Lactate + NAD+. Note the temperature of this reaction will not affect the standard reducton potential delta E'o in the table 13-7b. please provide a comprehensive explanation with each step taken
Begining with 1 M concentrations of each reactant and product at pH=7 and 25.0 degrees C, calculate the K'eq (to one decimal point) of the reaction Pyruvate + NADH+H+ <=> Lactate + NAD+. Note the temperature of this reaction will not affect the standard reducton potential delta E'o in the table 13-7b. please provide a comprehensive explanation with each step taken
Biochemistry
6th Edition
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Reginald H. Garrett, Charles M. Grisham
Chapter18: Glycolysis
Section: Chapter Questions
Problem 12P
Related questions
Question
|
Begining with 1 M concentrations of each reactant and product at pH=7 and 25.0 degrees C, calculate the K'eq (to one decimal point) of the reaction Pyruvate + NADH+H+ <=> Lactate + NAD+. please provide a comprehensive explanation with each step taken. |
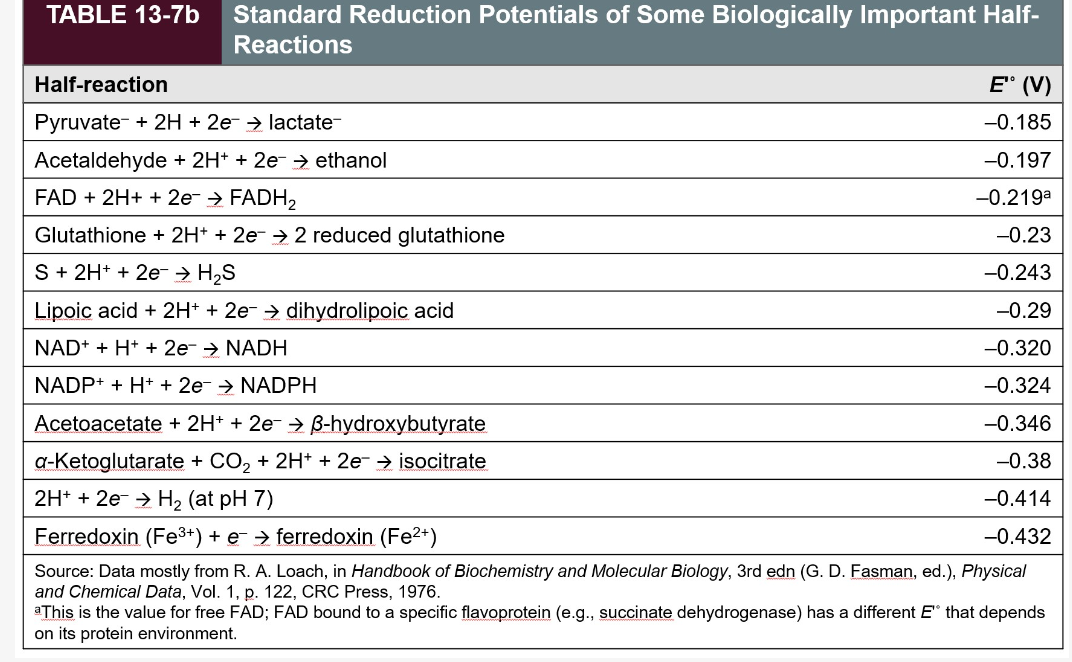
Transcribed Image Text:TABLE 13-7b
Standard Reduction Potentials of Some Biologically Important Half-
Reactions
Half-reaction
E" (V)
Pyruvate + 2H+ 2e → lactate-
-0.185
Acetaldehyde + 2H+ + 2e → ethanol
-0.197
FAD + 2H+ + 2e- → FADH₂
-0.219a
Glutathione + 2H+ + 2e → 2 reduced glutathione
-0.23
S + 2H+ + 2e → H₂S
-0.243
Lipoic acid + 2H+ + 2e → dihydrolipoic acid
-0.29
NAD+ + H+ + 2e → NADH
-0.320
NADP+ + H+ + 2e- → NADPH
-0.324
-0.346
Acetoacetate + 2H+ + 2e → B-hydroxybutyrate
a-Ke glutarate + CO₂ + 2H+ + 2e → isocitrate
-0.38
2H+ + 2e → H₂ (at pH 7)
-0.414
Ferredoxin (Fe³+) + e- → ferredoxin (Fe²+)
-0.432
Source: Data mostly from R. A. Loach, in Handbook of Biochemistry and Molecular Biology, 3rd edn (G. D. Fasman, ed.), Physical
and Chemical Data, Vol. 1, p. 122, CRC Press, 1976.
This is the value for free FAD; FAD bound to a specific flavoprotein (e.g., succinate dehydrogenase) has a different E" that depends
on its protein environment.

Transcribed Image Text:TABLE 13-7a Standard Reduction Potentials of Some Biologically Important Half-
Reactions
Half-reaction
E° (V)
¹/2 O₂ + 2H+ + 2e- → H₂O
0.816
Fe³+ + e Fe²+
0.771
NO3 + 2H+ + 2e → NO₂ + H₂O
0.421
Cytochrome f (Fe³+) + e- → cytochrome f (Fe²+)
0.365
Fe(CN)63- (ferricyanide) + e- → Fe(CN)64-
0.36
0.35
Cytochrome a3 (Fe³+) + ¯ → cytochrome a3 (Fe²+)
O₂ + 2H+ + 2e → H₂O₂
0.295
0.29
0.254
Cytochrome a (Fe³+) + e- → cytochrome a (Fe²+)
Cytochrome c (Fe³+) + e- cytochrome c (Fe²+)
Cytochrome (Fe³+) + e- → cytochrome c₁ (Fe²+)
Cytochrome b (Fe³+) + e- → cytochrome b (Fe²+)
Ubiquinone + 2H+ + 2e¯ → ubiquinol
0.22
0.077
0.045
Fumarate² + 2H+ + 2e → succinate2-
0.031
0.000
2H+ + 2e → H₂ (at standard conditions, pH 0)
Crotonyl-CoA + 2H+ + 2e → butyryl-CoA
-0.015
Oxaloacetate²- + 2H+ + 2e → malate²-
-0.166
Source: Data mostly from R. A. Loach, in Handbook of Biochemistry and Molecular Biology, 3rd edn (G. D. Fasman, ed.), Physical
and Chemical Data, Vol. 1, p. 122, CRC Press, 1976.
This is the value for free FAD; FAD bound to a specific flavoprotein (e.g., succinate dehydrogenase) has a different Eº that depends
on its protein environment.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning