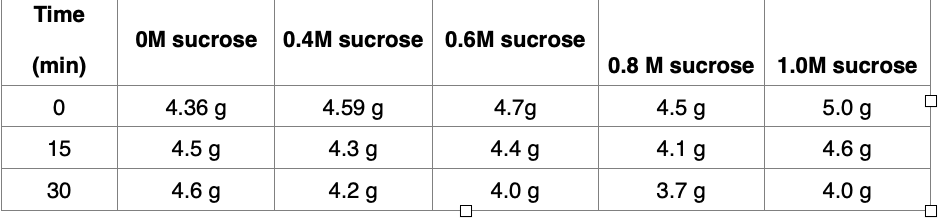
Below is a simple set of weights obtained while immersing potato slices in various sugar solutions over 30 minutes. I need help calculating all of this for a 30 min period a. For each solution, calculate the rate of weight change over this 30-minute period. Show your work in the space below. Once you calculate these rates - fill in the blanks below and then add these answers to your Canvas exam. Write your answer in standard notation and use 3 digits past the decimal point - e.g. 0.005, 0.030. (Note that the last zero in an answer is considered to be a digit). Show your work in the space below. 0.0 M sucrose = _______ _____________ g/min 0.4 M sucrose = _______ ____________ g/min 0.6 M sucrose = ________ ____________ g/min 0.8 M sucrose = ____________________ g/min 1.0 M sucrose = ________ __________ g/min Using the osmosis data in the question above, determine the percent weight change for each sucrose solution and fill in the blanks given below and then add these answers to your Canvas exam. Write your answer in standard notation and use 1 digit past the decimal point - e.g. 8.6. (Note that the last zero in the answer is considered to be a digit) Show your work in the space below. 0.0 M sucrose = _________ __________% 0.4 M sucrose = _______ ______________% 0.6 M sucrose = ___________________% 0.8 M sucrose = _____________________% 1.0 M sucrose = ____________________%
Below is a simple set of weights obtained while immersing potato slices in various sugar solutions over 30 minutes.
I need help calculating all of this for a 30 min period
a. For each solution, calculate the rate of weight change over this 30-minute period. Show your work in the space below. Once you calculate these rates - fill in the blanks below and then add these answers to your Canvas exam. Write your answer in standard notation and use
3 digits past the decimal point - e.g. 0.005, 0.030. (Note that the last zero in an answer is considered to be a digit). Show your work in the space below.
- 0.0 M sucrose = _______ _____________ g/min
- 0.4 M sucrose = _______ ____________ g/min
- 0.6 M sucrose = ________ ____________ g/min
- 0.8 M sucrose = ____________________ g/min
- 1.0 M sucrose = ________ __________ g/min
Using the osmosis data in the question above, determine the percent weight change for each sucrose solution and fill in the blanks given below and then add these answers to your Canvas exam. Write your answer in standard notation and
use 1 digit past the decimal point - e.g. 8.6. (Note that the last zero in the answer is considered to be a digit)
Show your work in the space below.
- 0.0 M sucrose = _________ __________%
- 0.4 M sucrose = _______ ______________%
- 0.6 M sucrose = ___________________%
- 0.8 M sucrose = _____________________%
- 1.0 M sucrose = ____________________%
Trending now
This is a popular solution!
Step by step
Solved in 2 steps









