
Chemistry & Chemical Reactivity
9th Edition
ISBN: 9781133949640
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 14, Problem 56GQ
Data in the table were collected at 540 K for the following reaction:
CO(g) + NO2(g) → CO2(g) + NO(g)
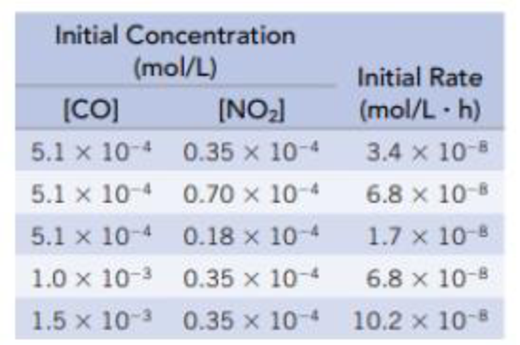
Using the data in the table:
- (a) Determine the reaction order with respect to each reactant.
- (b) Derive the rate equation.
- (c) Calculate the rate constant, giving the correct units for k.
Expert Solution & Answer
Trending nowThis is a popular solution!

Chapter 14 Solutions
Chemistry & Chemical Reactivity
Ch. 14.1 - Sucrose decomposes to fructose and glucose in acid...Ch. 14.1 - What are the relative rates of appearance or...Ch. 14.1 - Prob. 1RCCh. 14.1 - 2. Use the graph provided in Example 14.1 to...Ch. 14.2 - 1. Which of the following will not usually...Ch. 14.3 - The initial rate ( [NO]/ t] of the reaction of...Ch. 14.3 - The rate constant, k, at 25 C is 0.27/h for the...Ch. 14.3 - The reaction NO(g) + 1/2 Cl2(g) NOCl(g) is...Ch. 14.4 - Sucrose, a sugar, decomposes in acid solution to...Ch. 14.4 - Gaseous azomethane (CH3N2CH3) decomposes to ethane...
Ch. 14.4 - Prob. 3CYUCh. 14.4 - The catalyzed decomposition of hydrogen peroxide...Ch. 14.4 - Americium is used in smoke detectors and in...Ch. 14.4 - The decomposition of N2O5 is a first-order...Ch. 14.4 - Which of the following will confirm that the...Ch. 14.4 - 3. The equation for the decomposition of NO2(g) at...Ch. 14.5 - Prob. 1CYUCh. 14.5 - The colorless gas N2O4, decomposes to the brown...Ch. 14.5 - Prob. 1RCCh. 14.5 - Prob. 2RCCh. 14.6 - Nitrogen monoxide is reduced by hydrogen to give...Ch. 14.6 - Prob. 2CYUCh. 14.6 - One possible mechanism for the decomposition of...Ch. 14.6 - The rate equation for a reaction A + B C was...Ch. 14.6 - A reaction is believed to occur by the following...Ch. 14.6 - Prob. 1QCh. 14.6 - Prob. 2QCh. 14.6 - Prob. 3QCh. 14.6 - Prob. 4QCh. 14.6 - Prob. 5QCh. 14.6 - Determine the activation energy for the reaction...Ch. 14 - Give the relative rates of disappearance of...Ch. 14 - Give the relative rates of disappearance of...Ch. 14 - In the reaction 2 O3(g) 3 O2(g), the rate of...Ch. 14 - In the synthesis of ammonia, if [H2]/t = 4.5 104...Ch. 14 - Experimental data are listed here for the reaction...Ch. 14 - 6. Phenyl acetate, an ester, reacts with water...Ch. 14 - Using the rate equation Rate = k[A]2[B], define...Ch. 14 - A reaction has the experimental rate equation Rate...Ch. 14 - The reaction between ozone and nitrogen dioxide at...Ch. 14 - Nitrosyl bromide, NOBr, is formed from NO and Br2:...Ch. 14 - The data in the table are for the reaction of NO...Ch. 14 - The reaction 2 NO(g) + 2 H2(g) N2(g) + 2 H2O(g)...Ch. 14 - Data for the reaction NO(g) + O2(g) NO2(g) are...Ch. 14 - Data for the following reaction are given in the...Ch. 14 - The rate equation for the hydrolysis of sucrose to...Ch. 14 - The decomposition of N2O5 in CCl4 is a first-order...Ch. 14 - The decomposition of SO2Cl2 is a first-order...Ch. 14 - The conversion of cyclopropane to propene (Example...Ch. 14 - Hydrogen peroxide, H2O2(aq), decomposes to H2O()...Ch. 14 - The decomposition of nitrogen dioxide at a high...Ch. 14 - At 573 K, gaseous NO2(g) decomposes, forming NO(g)...Ch. 14 - The dimerization of butadiene, C4H6, to form...Ch. 14 - The decomposition of ammonia on a metal surface to...Ch. 14 - Hydrogen iodide decomposes when heated, forming...Ch. 14 - The rate equation for the decomposition of N2O5...Ch. 14 - Gaseous azomethane, CH3N=NCH3, decomposes in a...Ch. 14 - The decomposition of SO2Cl2 SO2Cl2(g) SO2(g) +...Ch. 14 - The compound Xe(CF3)2 decomposes in a first-order...Ch. 14 - The radioactive isotope 64Cu is used in the form...Ch. 14 - Radioactive gold-198 is used in the diagnosis of...Ch. 14 - Prob. 31PSCh. 14 - Ammonia decomposes when heated according to the...Ch. 14 - Gaseous NO2 decomposes at 573 K. NO2(g) NO(g) + ...Ch. 14 - The decomposition of HOF occurs at 25 C. HOF(g) ...Ch. 14 - Prob. 35PSCh. 14 - Prob. 36PSCh. 14 - Calculate the activation energy, Ea, for the...Ch. 14 - If the rate constant for a reaction triples when...Ch. 14 - When healed lo a high temperature, cyclobutane,...Ch. 14 - When heated, cyclopropane is converted to propene...Ch. 14 - The reaction of H2 molecules with F atoms H2(g) +...Ch. 14 - Prob. 42PSCh. 14 - What is the rate law for each of the following...Ch. 14 - What is the rate law for each of the following...Ch. 14 - Ozone, O3, in the Earths upper atmosphere...Ch. 14 - The reaction of NO2(g) and CO(g) is thought to...Ch. 14 - A proposed mechanism for the reaction of NO2 and...Ch. 14 - The mechanism for the reaction of CH3OH and HBr is...Ch. 14 - A reaction has the following experimental rate...Ch. 14 - For a first-order reaction, what fraction of...Ch. 14 - Prob. 51GQCh. 14 - Data for the following reaction are given in the...Ch. 14 - Formic acid decomposes at 550 C according to the...Ch. 14 - Isomerization of CH3NC occurs slowly when CH3NC is...Ch. 14 - When heated, tetrafluoroethylene dimerizes to form...Ch. 14 - Data in the table were collected at 540 K for the...Ch. 14 - Ammonium cyanate, NH4NCO, rearranges in water to...Ch. 14 - Prob. 58GQCh. 14 - At temperatures below 500 K, the reaction between...Ch. 14 - Nitryl fluoride can be made by treating nitrogen...Ch. 14 - The decomposition of dinitrogen pentaoxide N2O5(g)...Ch. 14 - The data in the table give the temperature...Ch. 14 - The decomposition of gaseous dimethyl ether at...Ch. 14 - The decomposition of phosphine, PH3, proceeds...Ch. 14 - The thermal decomposition of diacetylene, C4H2,...Ch. 14 - Prob. 66GQCh. 14 - The ozone in the Earths ozone layer decomposes...Ch. 14 - Hundreds of different reactions occur in the...Ch. 14 - Data for the reaction [Mn(CO)5(CH3CN)]+ + NC5H5 ...Ch. 14 - The gas-phase reaction 2 N2O5(g) 4 NO2(g) + O2(g)...Ch. 14 - Prob. 71GQCh. 14 - The decomposition of SO2Cl2 to SO2 and Cl2 is...Ch. 14 - The decomposition of nitrogen dioxide at a high...Ch. 14 - Prob. 74GQCh. 14 - Egg protein albumin is precipitated when an egg is...Ch. 14 - A The compound 1,3-butadiene (C4H6) forms...Ch. 14 - Hypofluorous acid, HOF, is very unstable,...Ch. 14 - We know that the decomposition of SO2Cl2 is...Ch. 14 - Nitramide, NO2NH2, decomposes slowly in aqueous...Ch. 14 - Prob. 80GQCh. 14 - Prob. 83ILCh. 14 - Prob. 84ILCh. 14 - The oxidation of iodide ion by the hypochlorite...Ch. 14 - The acid-catalyzed iodination of acetone...Ch. 14 - Prob. 87SCQCh. 14 - The following statements relate to the reaction...Ch. 14 - Chlorine atoms contribute to the destruction of...Ch. 14 - Prob. 91SCQCh. 14 - Prob. 92SCQCh. 14 - The reaction cyclopropane propene occurs on a...Ch. 14 - Prob. 94SCQCh. 14 - Examine the reaction coordinate diagram given...Ch. 14 - Draw a reaction coordinate diagram for an...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Determine [OH], [H+], and the pH of each of the following solutions. a. 1.0 M KCl b. 1.0 M KC2H3O2
Chemistry
Practice Problem 1.22 Which of the following alkenes can exist as cis-trans isomers? Write their structures. Bu...
Organic Chemistry
Describe the orbitals used in bonding and the bond angles in the following compounds: a. CH3O b. CO2 c. H2CO d....
Organic Chemistry (8th Edition)
The active ingredient in Tylenol and a host of other over-the-counter pain relievers is acetaminophen (C8H9NO2)...
Chemistry: Atoms First
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, & Biological Chemistry
Q2. Which statement best defines chemistry?
a. The science that studies solvents, drugs, and insecticides
b. Th...
Introductory Chemistry (5th Edition) (Standalone Book)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For a reaction involving the decomposition of a hypothetical substance Y, these data are obtained: Determine the order of the reaction. Write the rate law for the decomposition of Y. Calculate k for the experiment above.arrow_forwardAt 573 K, gaseous NO2(g) decomposes, forming NO(g) and O2(g). If a vessel containing NO2(g) has an initial concentration of 1.9 102 mol/L, how long will it take for 75% of the NO2(g) to decompose? The decomposition of NO2(g) is second-order in the reactant and the rate constant for this reaction, at 573 K, is 1.1 L/mol s.arrow_forwardOne possible mechanism for the decomposition of nitryl chloride, NO2CI, is What is the overall reaction? What rate law would be derived from this mechanism? What effect does increasing the concentration of the product NO2 have on the reaction rate?arrow_forward
- Sucrose, a sugar, decomposes in acid solution to give glucose and fructose. The reaction is first-order in sucrose, and the rate constant at 25 C is k = 0.21 h1. If the initial concentration of sucrose is 0.010 mol/L, what is its concentration after 5.0 h?arrow_forwardThe hydrolysis of the sugar sucrose to the sugars glucose and fructose, C12H22O11+H2OC6H12O6+C6H12O6 follows a first-order rate equation for the disappearance of sucrose: Rate =k[C12H22O11] (The products of the reaction, glucose and fructose, have the same molecular formulas but differ in the arrangement of the atoms in their molecules.) (a) In neutral solution, k=2.11011s1 at 27 C and 8.51011s1 at 37 C. Determine the activation energy, the frequency factor, and the rate constant for this equation at 47 C (assuming the kinetics remain consistent with the Arrhenius equation at this temperature). (b) When a solution of sucrose with an initial concentration of 0.150 M reaches equilibrium, the concentration of sucrose is 1.65107M . How long will it take the solution to reach equilibrium at 27 C in the absence of a catalyst? Because the concentration of sucrose at equilibrium is so low, assume that the reaction is irreversible. (c) Why does assuming that the reaction is irreversible simplify the calculation in pan (b)?arrow_forwardFor a reaction involving the decomposition of Z at a certain temperature, the following data are obtained: (a) What is the order of the reaction? (b) Write the rate expression for the decomposition of Z. (c) Calculate k for the decomposition at that temperature.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
Kinetics: Chemistry's Demolition Derby - Crash Course Chemistry #32; Author: Crash Course;https://www.youtube.com/watch?v=7qOFtL3VEBc;License: Standard YouTube License, CC-BY