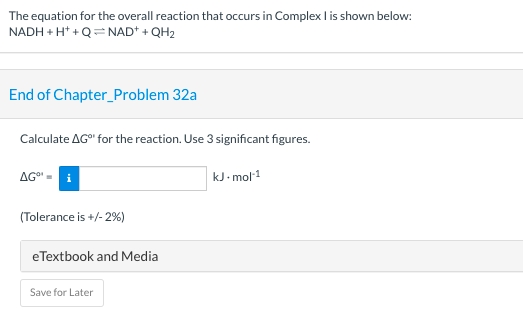
The equation for the overall reaction that occurs in Complex I is shown below: NADH + H* +Q= NAD+ + QH2 End of Chapter_Problem 32a Calculate AG for the reaction. Use 3 significant figures. AG" kJ- mol1 (Tolerance is +/- 2%) eTextbook and Media Save for Later
Q: Which nucleotide is shown in the picture above?
A: A nucleotide is formed by nitrogenous base, sugar and phosphate. Sugar binds with nitrogenous base b...
Q: WHAT ARE THE DIFFERENCES OF MONOSACCHARIDES, DISACCHARIDES AND POLYSACCHARIDES IN TERMS OF: STRUCTUR...
A: Carbohydrates are macromolecules composed of carbon, hydrogen, and oxygen. Carbohydrates are classif...
Q: Thioesters play important roles in glycolysis and tca cycle. List which reactions involve thioesters...
A: Thioesters are involved in a wide range of reactions. Attack on the carbonyl carbon to produce a tet...
Q: ased on the following qualitative tests for proteins, which one is positive/negative for intact prot...
A: Amino acid: Amino acids are building blocks of proteins and also act as an intermediate in metaboli...
Q: Choline test procedure (chemical reagents added), positive results/observations, and positive for (p...
A: Choline is an essential nutrient for humans and animals. Choline occurs in the form of ions that for...
Q: Enzymes are catalysts that increase the rate of reactions by pulling two substrates together. low...
A: Catalysts speed up the chemical reaction without being changed themselves. All living organisms prep...
Q: Define triad
A: Those organic molecules are mainly comprised of two functional groups; an amino group and a carboxyl...
Q: WHAT ARE THE ADVANTAGES AND DISADVANTAGES OF CARBOHYDRATES IN BIOCHEMISTRY? GIVE AT LEAST 3 FOR EACH...
A: Carbohydrates are the one of the biomolecules that provide energy to the human body. They are mainly...
Q: Please answer in detail ASAP
A: Carbohydrates are macronutrients and one of the three basic sources of energy for our bodies. They a...
Q: What is your opinion/stance on biofuels as an alternative energy source?
A: Definition: Biofuels are described as fuels made from biomass in a broad sense (matter derived from ...
Q: You are working in a research lab that is surveying the CFTR gene sequence in humans to identify new...
A: A synonymous mutation is mutation in the DNA sequence that leads to change in the codon for amino ac...
Q: What is the concepts of the native conformation of proteins? Why and how do proteins refold and unfo...
A: Proteins are polypeptide structures consisting of one or more long-chain amino acid residues. They a...
Q: All of the following is correct about folate and vitamin B12 involvement in resynthesis of methionin...
A: Vit B12 and folate have a very important role in many different physiological functions. Vit B12 and...
Q: DATA AND RESULT: Hydrolysis of Disaccharides and Polysaccharides Sucrose + H,O Sucrose + HCI Starch ...
A: There are various kind of test to identify mono- di- oligi- and polysaccharide. It depends on its re...
Q: Hydrolysis of Disaccharides and Polysaccharides Sucrose + H,O Sucrose + HCI Starch + H,O Starch + HC...
A: The disaccharides and polysaccharides are broken into their component sugar molecules by the process...
Q: easure the element carbon (C) content using XRF?
A: XRF has limitations on the elements that can be measured. Elements lighter than magnesium can not be...
Q: 1. What is the name we give to structures which are not complete polypeptide chains but form superse...
A: "Since you have asked multiple questions, we will solve the first four questions for you. If you wan...
Q: what is the importance of getting rf value in chromotography tools?
A: Chromatography: Chromatography is an analytical technique in which compounds in a mixture are separ...
Q: The following were obtained in a study of an enzyme known to follow Michaelis-Menten kinetics: R...
A: Enzymes are proteins which accelerate the rate of biochemical reactions. The Michaelis-Menten plot r...
Q: Does this protein absorb light at 280 nm? If yes, please write (as a number) how many residues contr...
A: Amino acids are organic compound with functional group namely carboxyl and amino. Proteins are forme...
Q: Consider a mixture of two proteins with molecular weights of 20,000 and 200,000. For simplicity of c...
A: Sedimentation Coefficient is the rate per unit centrifugal field experienced by the particle undergo...
Q: e tra led by the solvent Distance travellad bu
A: Chromatography is a technique by which we separate molecules from a mixture In this technique, we us...
Q: Describe how the following properties affect the function of a protien: A.) R group orientation B.) ...
A: Amino acids are biomolecules that are comprised of two functional groups, these are an amino group (...
Q: In UV/Visible spectrophotometer analysis for a multicomponent system, there are only two dyes used i...
A: UV/Visible Spectrophotometry detects the absorbance of light in the UV/Visible range of the electrom...
Q: Qualitative Analysis of Proteins Tests
A: Proteins are large biological molecule composed of amino acid, amino acids contain both amino (nh2) ...
Q: make a short paragraph what makes essential fatty acids essential?
A: Essential fatty acids, or EFAs, are fatty acids that humans and other animals must ingest because it...
Q: Briefly explain the Michaelis-Menten model of enzyme kinetics.
A: Enzymes are commonly composed of protein molecules that catalyze the biochemical reaction by decreas...
Q: Read through each scenario. Under the scenario, write which lab safety rule is being broken and indi...
A: Hi! Thank you for the question, as per the honor code, we are allowed to answer the first 3...
Q: Define active site
A: The enzymes speed up the reaction by several folds. Enzymes play a critical role in living systems. ...
Q: Define P/O ratio. State and explain the importance of P/O ratio.
A: P/O Ratio is also known as the phosphate/ Oxygen ratio.
Q: + J. Draw the structure of the following molecules and complete the table. Aldose/ Pentose/ Mono/Di/...
A: Carbohydrates: Carbohydrates are polyhydroxy aldehydes or ketones that are primarily produced by pl...
Q: What is the concentration of enzyme (in mM) needed to achieve a Vmax of 8.00 mM/s if the enzyme has ...
A:
Q: Which nucleotide is shown in the picture above?
A: A nucleotide is made up of a phosphate group, a sugar and a nitrogenous base. ATP provides energy to...
Q: HO, но. но NO2 G .F O,N" NO2 Br F 1. Provide the mechanism for the chemical reaction of pigment F wi...
A: The pigment in the question are Azo pigment/dye and is organic compound contains Azo group (-N=N-). ...
Q: The quality problem A central theme in biochemistry is that protein function is determined by prote...
A: Proteins have four levels of conformation. They are, the primary structure, secondary structure, ter...
Q: Given the statements identify which are correct or incorrect
A: Multiple subparts asked. I will answer the first 3 subparts; following guidelines. Ketose - Monosacc...
Q: Phospholipids spontaneously form a bilayer in an aqueous solution. Why do the heads of the phospholi...
A: The basic material for studying biological membranes has been red blood cells. Lipid and protein are...
Q: 1. How many different tripeptides are possible? How many carboxyl terminals of polypeptide chains ar...
A: Tripeptides are composed of three amino acids. There are 20 naturally occurring amino acids . A mol...
Q: 1. What are the ground electronic configurations of Ti and Ti* ion? Ti Tit
A:
Q: What are the factors that affect carbohydrates?
A: Nutrients are those compounds that are present in the food that living beings intake for better and ...
Q: If a protein were placed in a nonpolar solvent, would it have the same structure as the same protein...
A: Proteins play out an incredible assortment of particular and fundamental functions in the living cel...
Q: Between direct and indirect allosteric kinase inhibitors, which do you think requires a larger confo...
A: In Allosteric modulation of enzyme/protein function, a modulator molecule binds at a site other than...
Q: Define 'activation energy' of an enzyme catalysed single substrate reaction and mention the effects ...
A: Activation energy- The difference in free energy between the transition state and the reactants is c...
Q: What three components does a nucleotide consist of?
A: Nucleotides are the basic building block of nucleic acids. DNA and RNA are polymers of long chains o...
Q: Connection between Electron Transport & Phosphorylation Task: 1. Define P/O ratio 2. Explain its...
A: Our body is always working, continuously doing various metabolic activities even when we are sleepin...
Q: One of the main sources of sphingosine in the body is in the cell membrane. What complication could ...
A: Ceramide: These are the type of lipid molecules composed of sphingosine and fatty acids and are foun...
Q: Explain how the carbonate-bicarbonate buffer system works in balancing acid-base in the blood.
A: Buffers are solutions that have weak acid and its conjugate base. They nullify small changes in the...
Q: Glyceraldehyde is the only three-carbon aldotetrose, and it can exist as two stereoisomers. What is ...
A: We'll answer the first question since the exact one wasn't specified. Please submit a new question s...
Q: It is estimated that the most common amino acid in a protein is leucine. What is the relative leucin...
A: Leucine is an aliphatic hydrophobic amino acid residue. These residues populate the interior hydroph...
Q: What are some examples of biochemical tests for proteins that involve acid hydrolysis or base hydrol...
A: Proteins: Proteins are complex high-molecular-weight molecules that consist of amino acids joined b...
- Gibbs free energy is a measure of enthalpy taking entropy into account.
- It allows us to relate the two factors at a given temperature to find if the reaction is spontaneous.
- If Gibbs is negative, the reaction will occur and if positive it will not happen ().
- The change in Gibbs free energy is related to the emf of a redox reaction by the equation .
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

- Begining with 1 M concentrations of each reactant and product at pH=7 and 25.0 degrees C, calculate the K'eq of the reaction Pyruvate + NADH <=> Lactate + NADH+H+.Note the temperature of this reaction will not affect the standard reducton potential delta E'o in the table 13-7b.Begining with 1 M concentrations of each reactant and product at pH=7 and 25.0 degrees C, calculate the K'eq of the reaction Pyruvate + NADH Lactate + NADH+H+. Note the temperature of this reaction will not affect the standard reducton potentialA mutant version of DADH can use NADP+ as a cofactor for isopropanol oxidation. Velocity data was collected from reactions at a series of NADP+ concentrations. The following trendline was obtained for a Lineweaver-Burk plot of the data: y = 0.00007x + 0.0014 Note that the NADP+ substrate concentrations are in mM and the reaction velocity was measured in nmol/min. Calculate the Km and Vmax for DADH with this substrate. Show your work.
- An enzyme that follows simple Michaelis–Menten kinetics has an initial reaction velocity of 10 µmol⋅min-1 when the substrate concentration is five times greater than the KM. What is the Vmax of this enzyme in µmol⋅min−1?The equation of the double reciprocal plot is y = 0.5294 x + 1.4960. What is the value of vmax (in M/s)? The substrate concentration is given in units of molarity (M) and reaction velocity has units of molarity per second (M/s). (Report to three significant figures)Calculate the biochemical standard cell potential for the oxidation of NADH by molecular oxygen O2 + 2NADH + 2 H+ → 2H2O + 2NAD+
- You make reaction progress curve by plotting absorbance vs time (seconds) and find the equation of the line to be y = -0.00235x + 0.7129. Calculate the U/µL and U/mL of lactate dehydrogenase activity in this fraction. The LDH activity is done identical to what is indicated in the lab manual. Show each step of the calculation from AU/time to M/min, to mol/min, to µmol/min to µmol/min/µL (=U/µL).The standard reduction potential for ubiquione (A or coenzyme Q) is .045 V, and the standard reduciton potential (E) for FAD is -0.219 V. Using these values, show that the oxidation for FADH2 by ubiquinone theoretically liberates enough energy to drive the synthesis of ATP. Faraday constant =96.48KJ/Vol delta G' standard for ATP Synthesis is +30.5 KJ/mol R=8.314 J/mol K=1.987 cal/mol KAn enzyme catalyzes a reaction at a velocity of 20 μmol/min when the concentration of substrate (S)is 0.01 M. The Km for this substrate is 1 × 10-5 M. Assuming that Michaelis-Menten kinetics arefollowed, what will the reaction velocity be when the concentration of S is 1 ×10-6 M?
- Substrate 1 is plotted on the following kinetic data, the Vmax and Km for Substrate 1 are 95 uM/sec and 10 µM, respectively. Draw a curve for the data you would expect to observe for a different substrate (Substrate 2) with a Km = 30 µM (assuming that both substrates give the same Vmax), if [Enzyme] = 0.05 µM, calculate kcat for Substrate 1 and what is the catalytic efficiency in units of M-1s-1?1.1)the following data duscribe an enzyme-catalyzed reaction(hydrolysis of cabobenzoxyglycyl-L-tryptophan) Plot these results using a lineweaver-Burk method, and determine values for Km and Vmax. substrate concenrate(mM) Velocity(mM.sec-1) 2,5 0.024 5 0.036 10 0.053 15 0.060 20 0.061 25 0.062A dialyzed pigeon liver extract will catalyze the conversion of acetyl-CoAto palmitate and CoASH if supplied with Mg2+, NADPH, ATP, HCO3-, andcitrate.(a) If H14CO3– is supplied, what compounds will become labeled (permanently or transiently) during the course of the reaction? In whatcompounds will 14C accumulate?(b) Explain the role of citrate in this reaction.

