Blast furnace produces pig iron of composition Fe 94%, Si2%, Mn 0.5% and C 3.5% by reduction smelting of iron ore, coke and limestone. The analysis is as follows: Iron ore : Fe20, 78%, SiO2 8%, Alz03 5%, MnO 2% H20 7% Coke : 86% C and 10% S and 4% Al,03; Amount is 600 Kg per ton of pig iron Limestone : pure CaCO, to produce a slag of 45% Cao Calculate: a) Amount of ore/ton of pig iron b) % of total Si 0z and of Mno reduced in the furnace c) Amount of slag/ton of pig iron and its % composition.
Blast furnace produces pig iron of composition Fe 94%, Si2%, Mn 0.5% and C 3.5% by reduction smelting of iron ore, coke and limestone. The analysis is as follows: Iron ore : Fe20, 78%, SiO2 8%, Alz03 5%, MnO 2% H20 7% Coke : 86% C and 10% S and 4% Al,03; Amount is 600 Kg per ton of pig iron Limestone : pure CaCO, to produce a slag of 45% Cao Calculate: a) Amount of ore/ton of pig iron b) % of total Si 0z and of Mno reduced in the furnace c) Amount of slag/ton of pig iron and its % composition.
Chapter26: Molecular Absorption Spectrometry
Section: Chapter Questions
Problem 26.15QAP
Related questions
Question
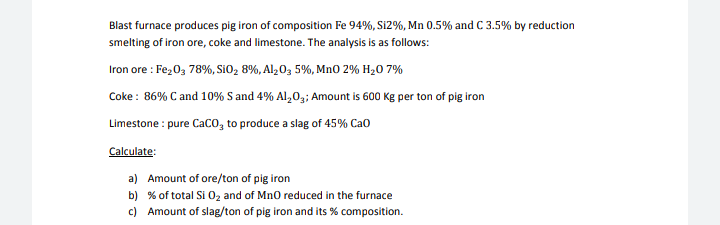
Transcribed Image Text:Blast furnace produces pig iron of composition Fe 94%, Si2%, Mn 0.5% and C 3.5% by reduction
smelting of iron ore, coke and limestone. The analysis is as follows:
Iron ore : Fe,0, 78%, SiO, 8%, Alz0, 5%, MnO 2% H20 7%
Coke : 86% C and 10% S and 4% Al,0,; Amount is 600 Kg per ton of pig iron
Limestone : pure Caco, to produce a slag of 45% Cao
Calculate:
a) Amount of ore/ton of pig iron
b) % of total Si 0z and of Mn0 reduced in the furnace
c) Amount of slag/ton of pig iron and its % composition.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning