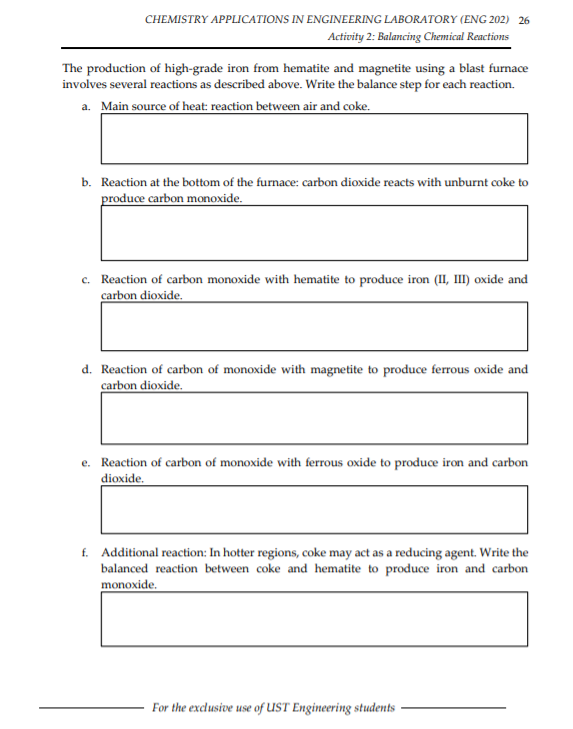
The production of high-grade iron from hematite and magnetite using a blast furnace involves several reactions as described above. Write the balance step for each reaction. a. Main source of heat: reaction between air and coke. b. Reaction at the bottom of the furnace: carbon dioxide reacts with unburnt coke to produce carbon monoxide. c. Reaction of carbon monoxide with hematite to produce iron (II, III) oxide and carbon dioxide. d. Reaction of carbon of monoxide with magnetite to produce ferrous oxide and carbon dioxide. e. Reaction of carbon of monoxide with ferrous oxide to produce iron and carbon dioxide. f. Additional reaction: In hotter regions, coke may act as a reducing agent. Write the balanced reaction between coke and hematite to produce iron and carbon monoxide.
The production of high-grade iron from hematite and magnetite using a blast furnace involves several reactions as described above. Write the balance step for each reaction. a. Main source of heat: reaction between air and coke. b. Reaction at the bottom of the furnace: carbon dioxide reacts with unburnt coke to produce carbon monoxide. c. Reaction of carbon monoxide with hematite to produce iron (II, III) oxide and carbon dioxide. d. Reaction of carbon of monoxide with magnetite to produce ferrous oxide and carbon dioxide. e. Reaction of carbon of monoxide with ferrous oxide to produce iron and carbon dioxide. f. Additional reaction: In hotter regions, coke may act as a reducing agent. Write the balanced reaction between coke and hematite to produce iron and carbon monoxide.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter20: Chemistry Of Selected Transition Elements And Coordination Compounds
Section: Chapter Questions
Problem 90QRT
Related questions
Question
100%
The production of high-grade iron from hematite and magnetite using a blast furnace involves several reactions as described
Transcribed Image Text:CHEMISTRY APPLICATIONS IN ENGINEERING LABORATORY (ENG 202) 26
Activity 2: Balancing Chemical Reactions
The production of high-grade iron from hematite and magnetite using a blast furnace
involves several reactions as described above. Write the balance step for each reaction.
a. Main source of heat: reaction between air and coke.
b. Reaction at the bottom of the furnace: carbon dioxide reacts with unburnt coke to
produce carbon monoxide.
c. Reaction of carbon monoxide with hematite to produce iron (II, III) oxide and
carbon dioxide.
d. Reaction of carbon of monoxide with magnetite to produce ferrous oxide and
carbon dioxide.
e. Reaction of carbon of monoxide with ferrous oxide to produce iron and carbon
dioxide.
f. Additional reaction: In hotter regions, coke may act as a reducing agent. Write the
balanced reaction between coke and hematite to produce iron and carbon
monoxide.
For the exclusive use of UST Engineering students
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax