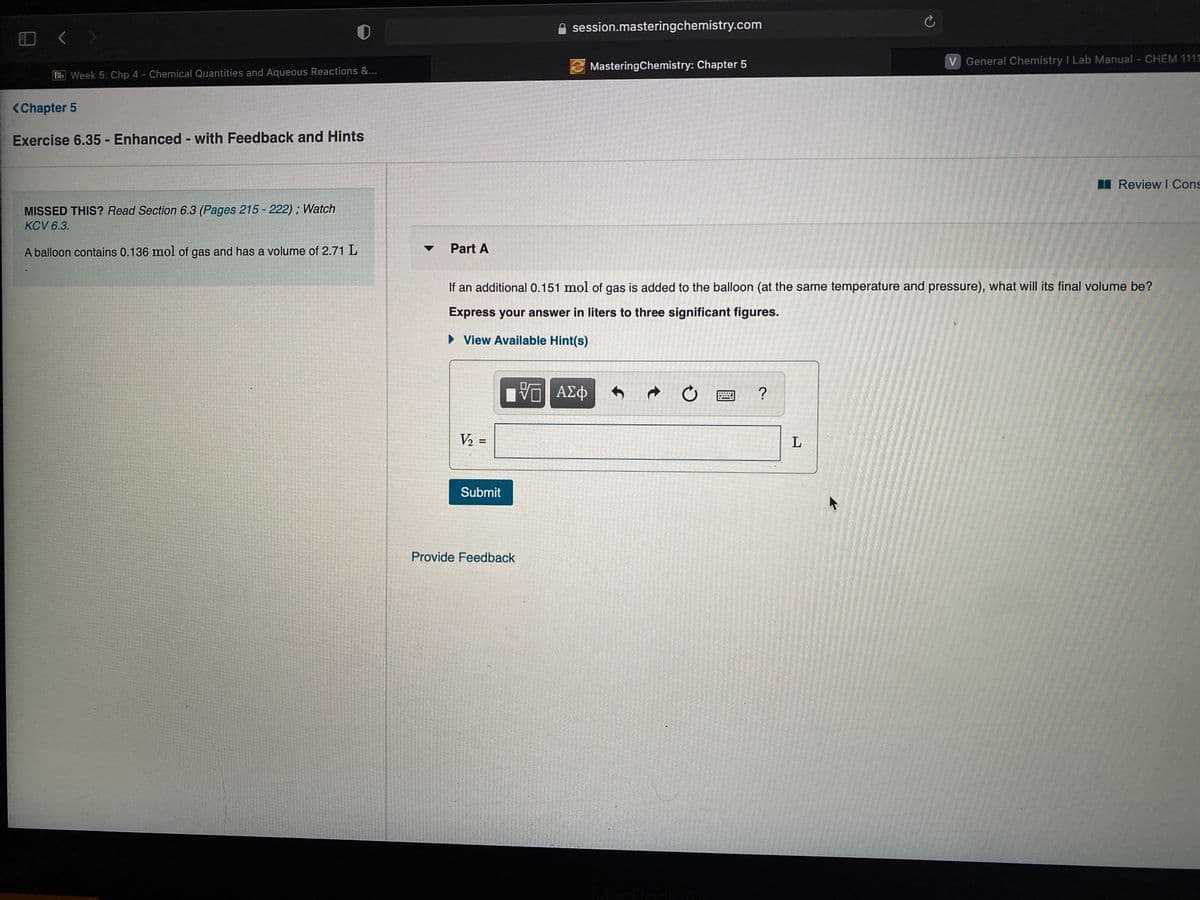
BN Review I Cons MISSED THIS? Read Section 6.3 (Pages 215 - 222) ; Watch KCV 6.3. A balloon contains 0.136 mol of gas and has a volume of 2.71 L Part A If an additional 0.151 mol of gas is added to the balloon (at the same temperature and pressure), what will its final volume be? Express your answer in liters to three significant figures. • View Available Hint(s) ? V2 = L Submit
BN Review I Cons MISSED THIS? Read Section 6.3 (Pages 215 - 222) ; Watch KCV 6.3. A balloon contains 0.136 mol of gas and has a volume of 2.71 L Part A If an additional 0.151 mol of gas is added to the balloon (at the same temperature and pressure), what will its final volume be? Express your answer in liters to three significant figures. • View Available Hint(s) ? V2 = L Submit
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.9QAP
Related questions
Question
Transcribed Image Text:session.masteringchemistry.com
3 MasteringChemistry: Chapter 5
V General Chemistry I Lab Manual - CHEM 1111
Bb Week 5: Chp 4 - Chemical Quantities and Aqueous Reactions &...
<Chapter 5
Exercise 6.35 - Enhanced- with Feedback and Hints
%3D
IReview I Cons
MISSED THIS? Read Section 6.3 (Pages 215 - 222); Watch
KCV 6.3.
A balloon contains 0.136 mol of gas and has a volume of 2.71 L
Part A
If an additional 0.151 mol of gas is added to the balloon (at the same temperature and pressure), what will its final volume be?
Express your answer in liters to three significant figures.
• View Available Hint(s)
ΑΣφ
V2 :
L
Submit
Provide Feedback
MacBook
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

