c. An Analytical laboratory at SMU has a bottle of concentrated HCI solution labelled, specific gravity of 1.2g/cm and which is 37% (w/w) HCI. M(HCI)= 36.46g/mol. From this 0.6mol,was carefully pipetted out into a 100ml standard flask and made up to the mark. Exactly 0.350g of pure sodium carbónate (Na CO3) M =105.988g/mol was dissolved into the above 100ml made up solution of HCI and the following reaction óccurred: Na2CO3 + 2HCI - 2NACI + CO2(g) + H20 i. What is the molar concentration of this HCI solution What is the resultant concentration of HCI in 100ml
c. An Analytical laboratory at SMU has a bottle of concentrated HCI solution labelled, specific gravity of 1.2g/cm and which is 37% (w/w) HCI. M(HCI)= 36.46g/mol. From this 0.6mol,was carefully pipetted out into a 100ml standard flask and made up to the mark. Exactly 0.350g of pure sodium carbónate (Na CO3) M =105.988g/mol was dissolved into the above 100ml made up solution of HCI and the following reaction óccurred: Na2CO3 + 2HCI - 2NACI + CO2(g) + H20 i. What is the molar concentration of this HCI solution What is the resultant concentration of HCI in 100ml
Chapter31: Introduction To Analytical Separations
Section: Chapter Questions
Problem 31.19QAP
Related questions
Question
Please help with Question 2 (c) i and ii
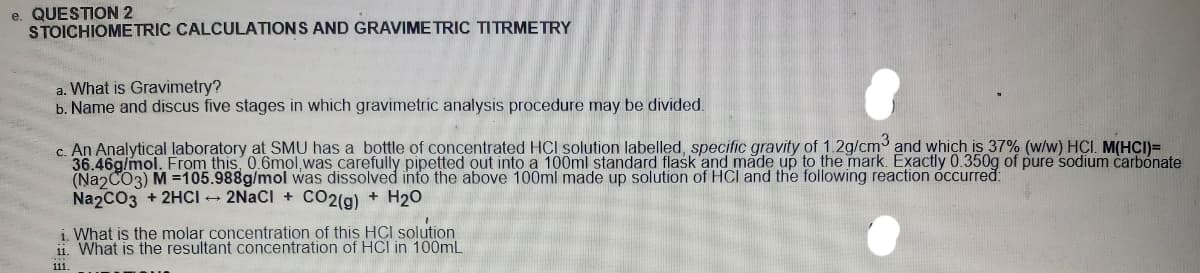
Transcribed Image Text:e. QUESTION 2
STOICHIOMETRIC CALCULATIONS AND GRAVIME TRIC TITRMETRY
a. What is Gravimetry?
b. Name and discus five stages in which gravimetric analysis procedure may be divided.
c. An Analytical laboratory at SMU has a bottle of concentrated HCI solution labelled, specific gravity of 1 2g/cm and which is 37% (w/w) HCI. M(HCI)=
36.46g/mol. From this 0.6mol, was carefully pipetted out into a 100ml standard flask and made up to the mark. Exactly 0.350g of pure sodium carbonate
(NaɔCO3) M =105.988g/mol was dissolved into the above 100ml made up solution of HCI and the following reaction óccurred:
Na2CO3 + 2HCI - 2NACI + CO2(g) + H20
i. What is the molar concetration of this HCI solution
11. What is the resultant concentration of HCI in 100mL
111.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



