C. In the buffer zone between 20 and 30 mL of titrant added the pH doesn't change very much. How does Le Chatelier's Principle explain this phenomenon? d. Using the pH at the start of the titration, find the K, for this acid. You may find it helpful to set up an ICE table as part of your solution
C. In the buffer zone between 20 and 30 mL of titrant added the pH doesn't change very much. How does Le Chatelier's Principle explain this phenomenon? d. Using the pH at the start of the titration, find the K, for this acid. You may find it helpful to set up an ICE table as part of your solution
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter15: Acid–base Equilibria
Section: Chapter Questions
Problem 99AP
Related questions
Question
100%
Part (c) and (d) only
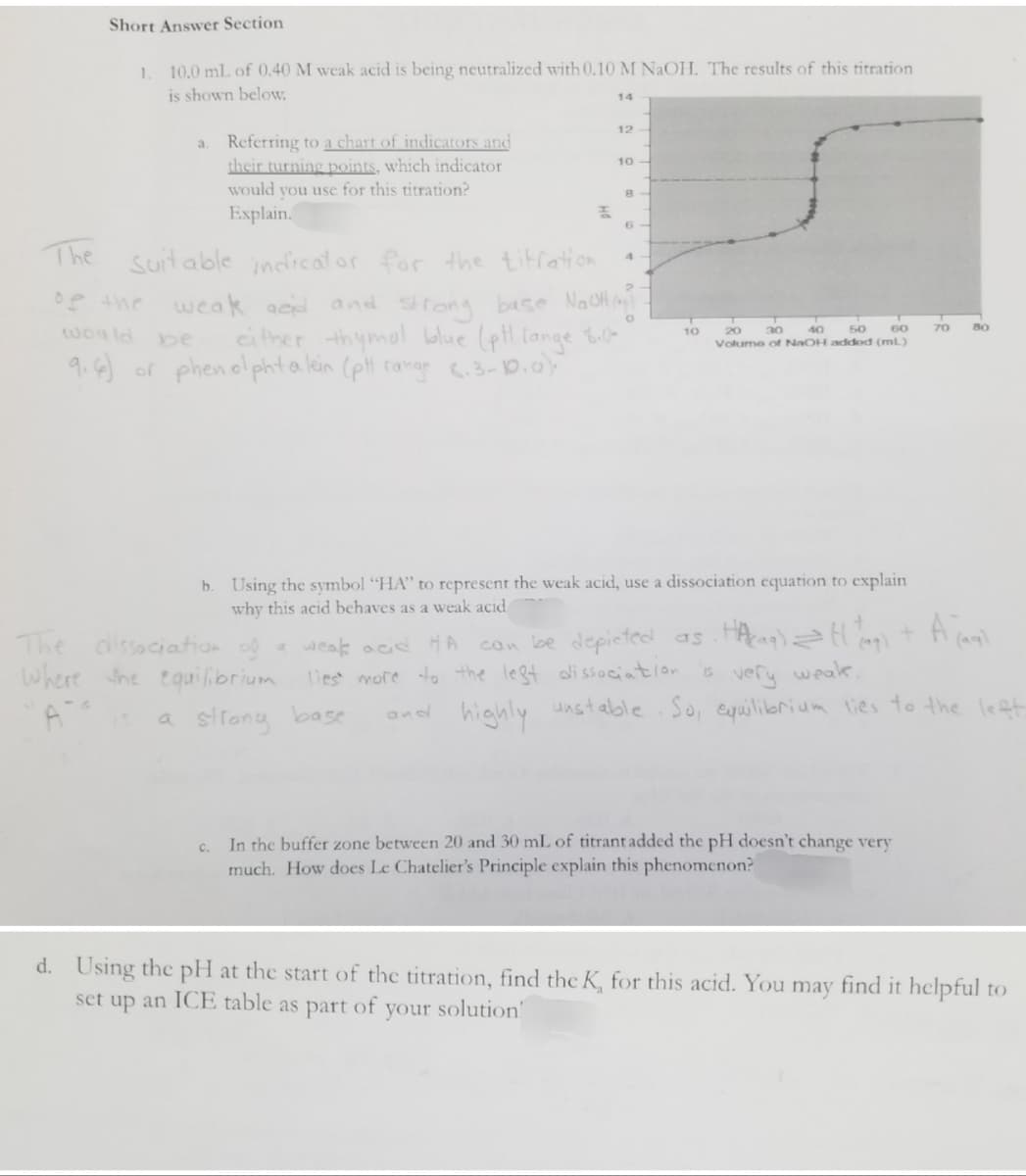
Transcribed Image Text:Short Answer Section
1. 10.0 mL of 0.40 M weak acid is being neutralized with 0.10 M NaOH. The results of this titration
is shown below.
14
12
a.
10
Referring to a chart of indicators and
their turning points, which indicator
would you use for this titration?
Explain.
8
F
6
The
4
Suitable indicator for the titration.
weak and and strong base NaOH (₂)
either thymol blue (pt lange 8.k
9.6) or phenolphta lein (pH range 6.3-10.0).
0
20
would be
50
70
40
30
60
Volume of NaOH addod (ml)
b. Using the symbol "HA" to represent the weak acid, use a dissociation equation to explain
why this acid behaves as a weak acid
A jaql
The dissociation of a weak acid HA
Where the equilibrium
a strong base
can be depicted as . Hacaq) = H (4₂) +
is very weak,
lies more to the left dissociation
and highly unstable. So, equilibrium lies to the left.
c. In the buffer zone between 20 and 30 mL of titrant added the pH doesn't change very
much. How does Le Chatelier's Principle explain this phenomenon?
d. Using the pH at the start of the titration, find the K, for this acid. You may find it helpful to
set up an ICE table as part of
your solution!
10
80
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning
