C. Total atomic orbitals for both neon atoms? d. How many molecular orbitals would the two neon atoms create? Write out what are they (e.g. bonding or antibonding for each orbital combination) e. How many core electrons and valence electrons does neon have? f. If two neon atoms come together how many electrons total would be present?
C. Total atomic orbitals for both neon atoms? d. How many molecular orbitals would the two neon atoms create? Write out what are they (e.g. bonding or antibonding for each orbital combination) e. How many core electrons and valence electrons does neon have? f. If two neon atoms come together how many electrons total would be present?
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter12: Chemical Bonding
Section: Chapter Questions
Problem 23CR: List the order in which the orbitals are filled as the atoms beyond hydrogen are built up. How many...
Related questions
Question
Please help part c,d,e,f,g and h
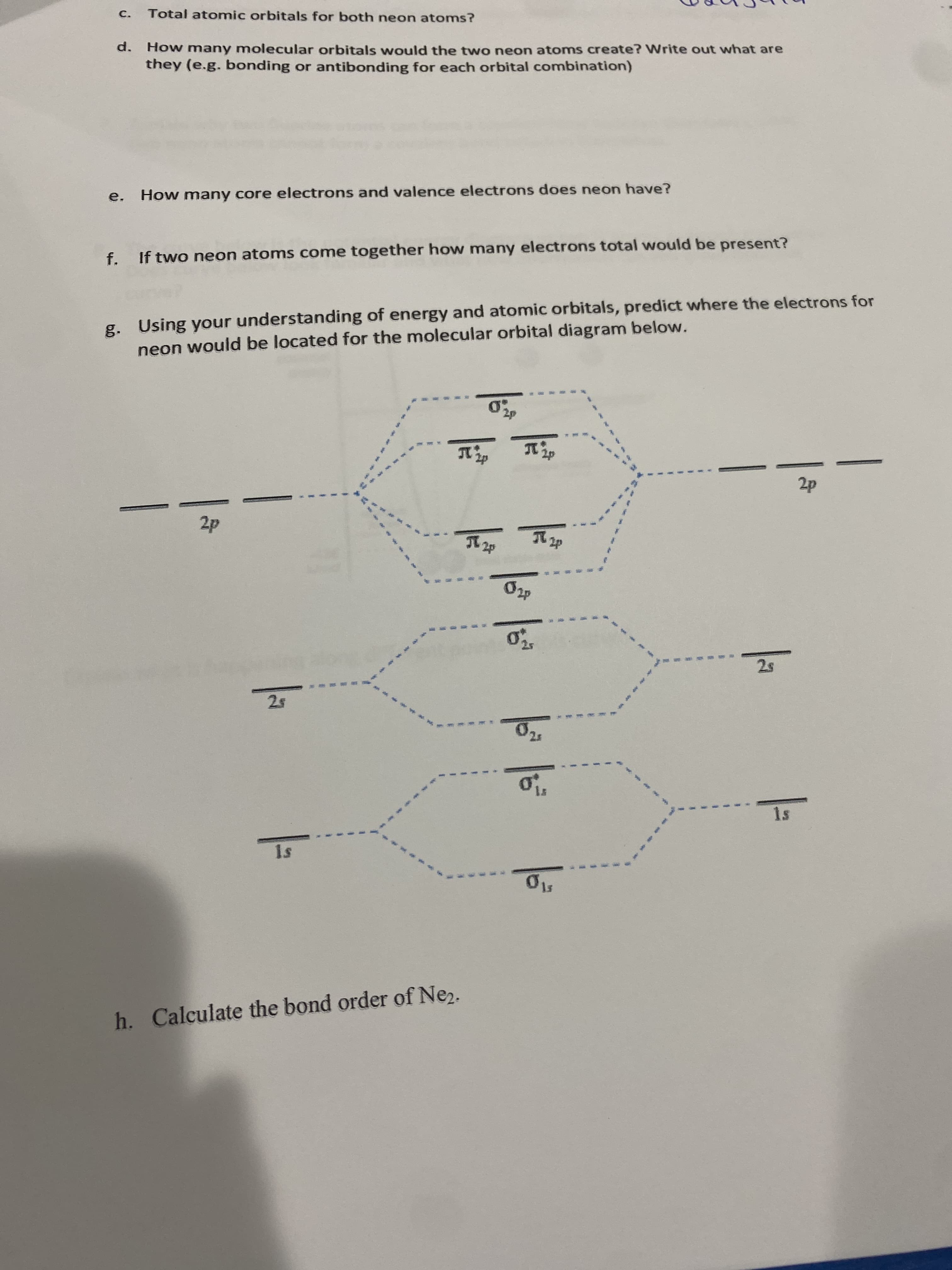
Transcribed Image Text:C.
Total atomic orbitals for both neon atoms?
d. How many molecular orbitals would the two neon atoms create? Write out what are
they (e.g. bonding or antibonding for each orbital combination)
e. How many core electrons and valence electrons does neon have?
f. If two neon atoms come together how many electrons total would be present?
g. Using your understanding of energy and atomic orbitals, predict where the electrons for
neon would be located for the molecular orbital diagram below.
1s
h. Calculate the bond order of Ne2.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning