Part A In the molecule FI, which atom is the negative pole? • View Available Hint(s) O F O I Part B Of the molecules HF and HCl, which has bonds that are more polar? • View Available Hint(s) О НF O HCI
Part A In the molecule FI, which atom is the negative pole? • View Available Hint(s) O F O I Part B Of the molecules HF and HCl, which has bonds that are more polar? • View Available Hint(s) О НF O HCI
Chapter5: Chemical Bonding
Section: Chapter Questions
Problem 46E: CH3COCH3 (acetone) is a common laboratory solvent that is often used in nail polish remover. Its...
Related questions
Question
Please answer question 3 part A, B, and C

Transcribed Image Text:Part C
All four of the silicon-chlorine single bonds in SiCl, are polar. In which direction should the polarity arrows point?
to the right
to the left
toward the central silicon atom
away from the central silicon atom
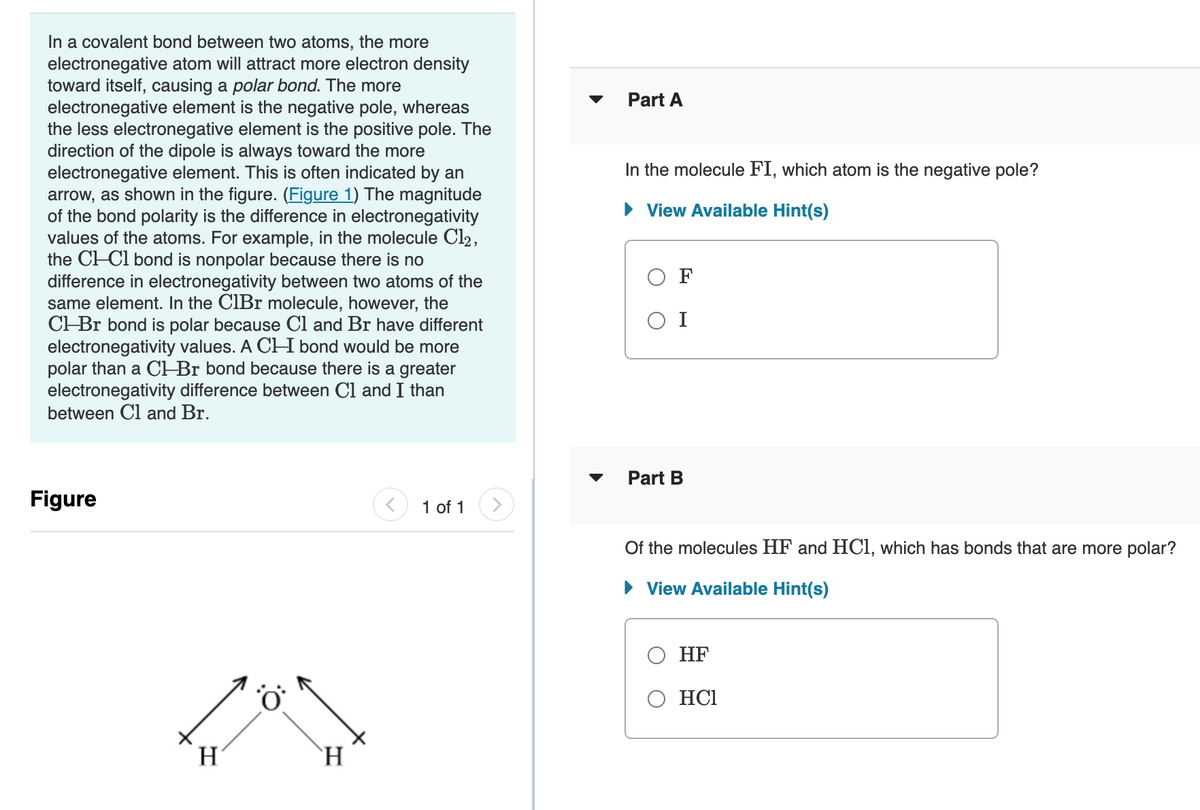
Transcribed Image Text:In a covalent bond between two atoms, the more
electronegative atom will attract more electron density
toward itself, causing a polar bond. The more
electronegative element is the negative pole, whereas
the less electronegative element is the positive pole. The
direction of the dipole is always toward the more
electronegative element. This is often indicated by an
arrow, as shown in the figure. (Figure 1) The magnitude
of the bond polarity is the difference in electronegativity
values of the atoms. For example, in the molecule Cl2,
the CH-Cl bond is nonpolar because there is no
difference in electronegativity between two atoms of the
same element. In the CIBr molecule, however, the
CHBr bond is polar because Cl and Br have different
electronegativity values. A CH bond would be more
polar than a CH Br bond because there is a greater
electronegativity difference between Cl and I than
between Cl and Br.
Part A
In the molecule FI, which atom is the negative pole?
• View Available Hint(s)
O F
O I
Part B
Figure
1 of 1
Of the molecules HF and HC1, which has bonds that are more polar?
• View Available Hint(s)
О НF
O HCl
H
`H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 2 images

Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning



Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning