C3H8 + 5 O2 → 3 CO2 + 4 H2O a) If I start with 5 grams of C3H8, what is my theoretical yield of water? Molar mass of C3H8 = 44.01g/mol Molar mass of H2O = 18.02 g/mol b) I got a percent yield of 75% How many grams of water did I make?
Q: When heated in the air, 1.63 g of zinc (Zn) combines with 0.40 g of Oxygen (O2) to form zinc oxide. ...
A:
Q: List combination of quantum numbers (n, l, ml) that could describe the size, shape, and orientation ...
A: The answer is as follows:
Q: Match the reservoir with the correct turnover rate of carbon: + land biosphere A. centuries + ocean ...
A: Turnover rate: fraction of material that leaves a reservoir in a specifed time interval. Mathematic...
Q: In an aqueous solution, classify these compounds as strong acids, weak acids, strong bases, weak bas...
A:
Q: Balancing PbCl2+AgNo3= Pb(No3)2+ AgCl
A: Balanced chemical equation: Balance chemical equation is the reaction in which number of atoms of al...
Q: The rate law for a reaction is rate = k[A][B][C][D]. The units of the rate constant when the concent...
A: Unit of rate constant can be determine from the rate law expression.
Q: Topic : PHASE e) What is difference between the triple point and critical point explain with exampl...
A: Part e :- Triple point :- In phase diagram it is defined as the point of particular temperature and...
Q: The activation energy for a particular reaction is 102 kJ/mol. If the rate constant is 1.35 × 10⁻⁴ s...
A:
Q: If K= 3.5 & K= 1.58 x 10 10, Suppose 100 ml of 0.010M aqueous amine is extracted with 200ml of benze...
A:
Q: c) Determine products A to C from the following reactions, some reaction may produce more than one p...
A: The products are as follows:
Q: Draw the expected major product for sequence MCPBA
A:
Q: You wanted to produce 2-nitrobenzene-1,4-dicarboxylic acid. For that purpose, you started he reactio...
A: The correct synthetic method applied here is :- Starting with alkyl benzene(toulene) which upon nit...
Q: .Suggest how to make syndiotactic/isotactic polystyrene on the one hand and on the other hand how to...
A: Step-I Isotactic polymers have the side groups attached on one side of the backbone chain whereas th...
Q: edictung uhe Try Again Your answer is incorrect. • Row 3: Your answer is incorrect. Predict the prod...
A: The balanced chemical reaction is given below : SO3(g) + 2H₂O(l) → H3O+ (aq) + HSO4- (aq) This...
Q: A 3.054 g sample of Vanadium was combined with oxygen to form 4.454 g of product. Calculate the empi...
A: To determine the emperical formula , we have to calculate moles of vanadium and and oxygen present i...
Q: What is the IUPAC name of this spectrum
A: IUPAC name have some specific rule
Q: topic: matter do you think that each sample occupies space? write the reason(s) for each sample wa...
A: Yes, it does because whatever we see when you open ur eyes is matter and what you feel when you clo...
Q: describes them. Color Density in g/cm Melting Point in Substance Kelvin Aluminum Oxidane Xenon Сoppe...
A: Fill in the table
Q: 100) 20 gm of binary electrolyte (mol. wt. = was dissolved in 500 gm water. Freezing point of soluti...
A: The degree of dissociation of electrolyte is given below
Q: How would you carry out the following synthesis? ÇCH3 SO3H
A: We have to synthesis p-sulphonicacetophenone from benzene. Since both sulphonic and aceto group both...
Q: The initial concentration of aqueous hydrogen peroxide, H 2O 2, was 0.1463 M; after decomposition fo...
A: The balanced reaction given is, => 2 H2O2 (aq) → 2 H2O (l) + O2 (g) Given: Initial concentration...
Q: Rank the gasses from highest to lowest global warming potential. (1 being the highest, 4 being the l...
A: The order would be : Carbon dioxide( CO2 ) > Methane (CH4) > Nitrous oxide(N2O) Thus ranking ...
Q: Draw the major product(s) of the following reaction. KMNO4 H20
A:
Q: Show the calculation steps that could be used to calculate the molality if given the mass of a solut...
A: Molality of solution can be defined as the number of moles of solute dissolved per kilogram of solve...
Q: Give a single benefit for each polymerisation from carrying them out under a nitrogen atmosphere? (...
A: Introduction: The polydispersity index (PDI) is dependent on the two parameters, i.e (I) number aver...
Q: 1) calculate the molar mass of ammonium thiosukfate
A: Since you have multiple questions as per guidelines we can answer one per session . If you want rema...
Q: 1. The decomposition of azomethane (C2H6N2) creates nitrogen gas and ethane gas (C2H6) with a rate c...
A:
Q: T0.03g 1. Mass of empty small evaporating dish (nonalpe noin2.24g 2. Mass of small evaporating dish ...
A: The mass of empty small evaporating dish is = 70.03 g The mass of small evaporating dish + mixture i...
Q: You are on a road trip. Your car travels at an average speed of 65 miles/hr, with an average gas use...
A: Given: Speed of the car = 65 miles/hr. Average gas usage = 17.8 Km/L. And time travelled = 2.6 hours...
Q: The analysis of a salt shows that it contains 56.58% potassium (K); 8.68% carbon (C) and 34.73% oxyg...
A: Given: Mass percent of K = 56.58 %. Mass percent of C = 8.68 % And mass percent of O = 34.73 %
Q: d) increasing the pressure in the reaction vessel e) decreasing the temperature of the system
A: For the preparation of Ammonia the haber process is scientifically used and is given by when one of ...
Q: C: Classify the specific ingredient of household substances as either Bronsted-Lowry acid or a Brons...
A: The compounds given are,
Q: what is the iupac name and structure of this spectrum
A: From The NMR spectrum we can see that their are of two type of hydrogen in the compound.
Q: A sample of 5 lactobacillus bacteria found in yogurt measure 7.9 x 10-6 m in total length. A second ...
A: Total length of 5 bacteria = 7.9 x 10-6 m Total length of 3 bacteria = 5.2 x 10-6 m
Q: An element consists of 4.35% of an isotope with mass 49.946 u, 83.79% of an isotope with mass 51.940...
A: Average atomic mass: The average atomic mass of an element is the sum of the masses of its isotopes,...
Q: A chemical reaction is characterized by the following experimental data: Rate Constant (L mol-'s-) T...
A:
Q: By convention, a chemical bond between two atoms with an electronegativity difference of 2.0 is whic...
A: Electronegativity is defined as tendency of atom to attract shared pair of electrons.
Q: In a calorimetry experiment similar to the one performed today, a student used 50.0 mL of 1.0 M NaOH...
A: Since you have posted multiple questions, we are entitled to answer the first only. 5) Given: Concen...
Q: Transformation of carbolic acid to o-hydroxybenzaldehyde. .cO.Me Protection Reduction Deprotection н...
A: It is quite necessary for any organic entity with multiple functionality to follow protection strate...
Q: a) Propose a synthesis route to prepare 3-methylhexan-3-ol from pentan-2-one. Note that more than on...
A: For a synthesis of compound 3-methylhexan-3-ol from pentan-2-one in two step reaction we follow a re...
Q: At 30°C, pure benze has a vapor pressure of 121.8 mmHg. Dissolving 15.0g of a non-volatile solute, R...
A: If a non-volatile solute is added to a solvent then according to Rault's law there is a relative low...
Q: 20. Draw Lewis dot structures showing the formation of an ionic compound from each of the following ...
A: Lewis dot structure of given compounds are as follows
Q: Brzy l 2 H2O s0yleat NMO
A:
Q: Fill in the gaps in the following table: Symbol P3- Protons 35 49 Neutrons 45 66 118 Electrons 46 76...
A:
Q: What is the value of the rate constant and what are the units for the rate constant? k = x 10-3 O L'...
A:
Q: QUESTION 1 1. The concentration of an unknown weak acid solution is 0.25M, and the solution has a pH...
A: pH , Ka and concentration related detail mathematical calculation is shown below
Q: How many ml of 4.00 M stock solution are needed to make 57.7 ml of 0.288 M solution?
A: in this we have to use dilution equation M1V1 = M2V2
Q: Consider the reaction, CH3CI(g) + 3CI2(g) → CCI4(g) + 3HCI(g) (a) Express the rate of reaction with ...
A: We are to write the rate of reaction for given reaction CH3Cl + 3Cl2 → CCl4 + 3HCl and, [HCl] = 0.02...
Q: Determine the E° , of a voltaic cell composed of Cr(s) in a 1.00M solution of Cr and Au(s) in a 1.00...
A:
Q: How many moles of solute particles are present in 1.43 mL of 0.447 M K2SO4? When you have your ans...
A: Number of solute particles can be calculated from the volume and concentration.
C3H8 + 5 O2 → 3 CO2 + 4 H2O
- a) If I start with 5 grams of C3H8, what is my theoretical yield of water?
Molar mass of C3H8 = 44.01g/mol
Molar mass of H2O = 18.02 g/mol
b) I got a percent yield of 75% How many grams of water did I make?
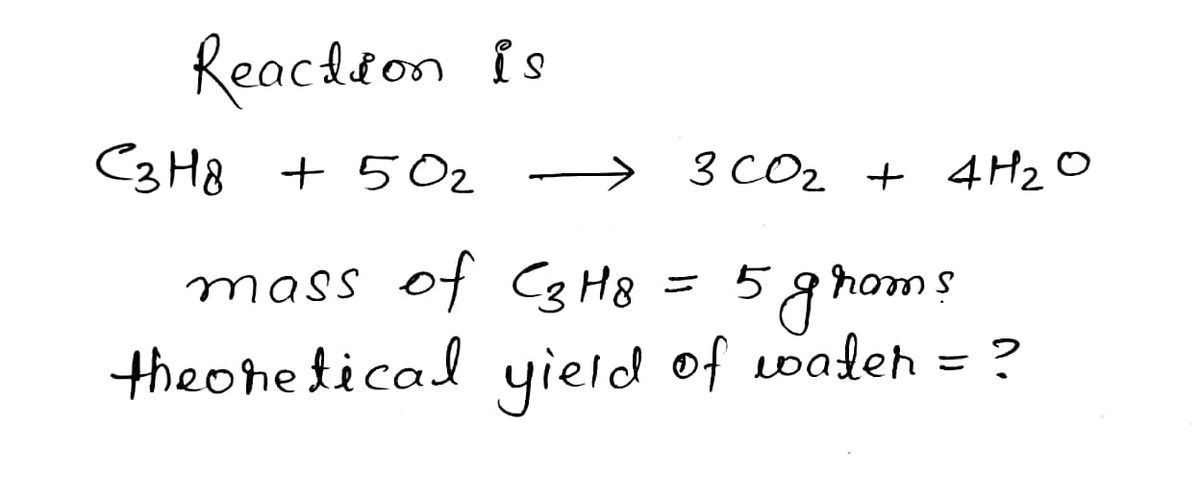
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

- 2Cr(OH)3 + 6HI -> 2CrI3 + 6H2O 1. If you start with 1000 g of HI, How many grams of H2O are formed? 2. If Actual yield of H2O is 700 g, What is the percent yield of H2O? 2C2H6 + 7O2 -> 4CO2 + 6H2O 1. How many grams of O2 are required to produce 10 moles of H2O? 2 Na + CI2 -> 2NaCI What mass of Na would be required to produce 12 moles of NaCI? Fe2O3 + 3CO -> 2Fe + 3CO2 If 2000 g of Fe2O3 reacts with 100 g of CO, how many moles of Fe would be produced? H2 + O2 -> H2O How many grams of H2 are needed to produce 72 grams of H2O? 4NH3 + 5O2 -> 4NO + 6H2O How many grams of NO form when a 25 g of NH3 is mixed with 32 g of O2?2 CuCl2 + 4 KI → 2 CuI + 4 KCl + I2 When 0.56 moles of CuCl2 reacts with 0.64 moles of KI, how many grams of I2 are formed?Consider the balanced chemical reaction: 2Al+3Br2-----2AlBr3 If 6.0 g of aluminum reacts with 68 g of bromine and the reaction has an 85% yield, how many grams of aluminum bromide can you form? a. Theoretical yield of AlBr3= ? b. Actual yield of AlBr3=?
- Preparation of Cr (accac)3 Reactants used: 0.26 g CrCl3 .6H20 1g of urea 0.8mL acetone Actual mass of Cr(acac3)/ Final Product =1.10g How would I calculate the percentage yield? I'm asking because I did it and got a yield that was more than 100%3NO2+H2O->2HNO3+NO How many grams of NO2 are needed to produce 254.5g of HNO3?When benzene (C6H6) reacts with bromine (Br)2, bromobenzene(C6H5Br) is obtained:C6H6 + Br2-------->C6H5Br + HBr(a) When 30.0 g of benzene reacts with 65.0 g of bromine, whatis the theoretical yield of bromobenzene? (b) If the actual yieldof bromobenzene is 42.3 g, what is the percentage yield?
- Part 1 How many grams of CO2 are produced when 2.50 grams of heptane is reacted withexcess oxygen? Equation: C7H16 + O2 -> H2O + CO2. part 2 What is the percent yield if 3.82 grams of CO2 are collected after the reaction of CO2are produced when 2.50 grams of heptane is reacted with excess oxygen? Equation:C7H16 + 02 -> H20 + CO2.If 25 grams of methane, CH4, and 50 g of ammonia, NH3, are combined w/ excess oxygen, how much methane or ammonia will be left when the reaction is finished? 2CH4 + 2NH3 + O2 -> 2HCN + 6H2OSuppose you want to yield 2 kg carbon dioxide. With a 90% yield, how much C8H18 (in L) will you need? ρ, C8H18: 0.703 kg/L 2 C8H18 + 25 O2 → 16 CO2 + 18 H2O
- 6 CO2 + 6 H2O -> C6H12O6 + 6 O2 How many grams of C6H12O6 can be produced from 828.70 grams of CO2? Remember not to round your molar masses. Round your final answer to 2 decimal places.The combustion of pentene (C5H10) in the presence of oxygen gas will produce carbon dioxide and water vapor according to the chemical equation: 2 C5H10 + 15 02-10 CO₂ + 10 H₂O. How many grams of water will be produced if 248 g of pentene is burned in the presence of 319 g of oxygen gas?10. The combustion of butane can be represented by the following unbalanced equation:C4H10 + O2------------> CO2 + H2OHow many moles of oxygen are necessary to obtain 40g of H2O? Immersive reader A) 2.86 moles of O2 B) 1.40 moles of O2 C) 2.2 moles of O2 D) 4.6 moles of O2 E) None of the above

