Calculate of the expected pH of the solution in beaker 3 after the addition of 20 mL of 1.0 M HCl. Beaker 3 volume: 100mL Beaker 3 (1.0M CH3CO2H & 1.0M NaCH3CO2) pH init: 4.73 Breaker 3 (1.0M CH3CO2H & 1.0M NaCH3CO2) pH final: 4.56
Calculate of the expected pH of the solution in beaker 3 after the addition of 20 mL of 1.0 M HCl. Beaker 3 volume: 100mL Beaker 3 (1.0M CH3CO2H & 1.0M NaCH3CO2) pH init: 4.73 Breaker 3 (1.0M CH3CO2H & 1.0M NaCH3CO2) pH final: 4.56
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter16: Reactions Between Acids And Bases
Section: Chapter Questions
Problem 16.52QE
Related questions
Question
Calculate of the expected pH of the solution in beaker 3 after the addition of 20 mL of 1.0 M HCl.
Beaker 3 volume: 100mL
Beaker 3 (1.0M CH3CO2H & 1.0M NaCH3CO2) pH init: 4.73
Breaker 3 (1.0M CH3CO2H & 1.0M NaCH3CO2) pH final: 4.56
Expert Solution
Step 1
Step 1:
From the given condition, initial pH of beaker 3 is 4.73.
The volume is 100 ml=0.1L
Solutions in the beaker are: 1.0M CH3COOH and 1.0M CH3COONa.
The initial moles are, calculated as follows:
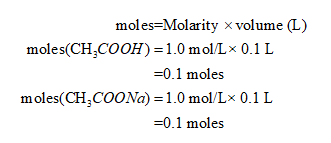
Thus, the Handerson Hasselbaach equation is applied as follows:
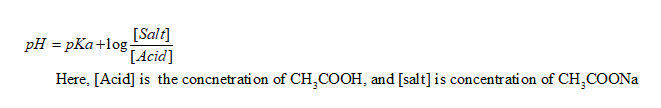
Now, putting the values it is obtained as:
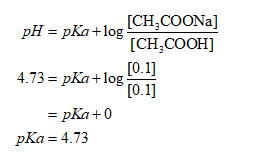
Thus, the pKa is obtained is 4.73.
Step by step
Solved in 2 steps with 7 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
