Calculate the binding energy for nucleus of a 4°Ca atom, answering the related questions along the way. Particle Relative Mass (u) 46Ca atom 45.953689 electron 5.485798 x 10-4 proton 1.007276 neutron 1.008665 u = 1.660539 x 10-27 kg C = 2.998 x 108 m/s %3D Periodic Table IMPORTANT: When entering your answers: • Use scientific notation • Enter the numbers only (no units) • Do not leave any spaces Report coefficients to the the 3rd decimal place (with proper rounding, regardless of the significant figures, use unrounde numbers in subsequent calculations) Report exponents as whole numbers (include the negative sign if necessary)
Calculate the binding energy for nucleus of a 4°Ca atom, answering the related questions along the way. Particle Relative Mass (u) 46Ca atom 45.953689 electron 5.485798 x 10-4 proton 1.007276 neutron 1.008665 u = 1.660539 x 10-27 kg C = 2.998 x 108 m/s %3D Periodic Table IMPORTANT: When entering your answers: • Use scientific notation • Enter the numbers only (no units) • Do not leave any spaces Report coefficients to the the 3rd decimal place (with proper rounding, regardless of the significant figures, use unrounde numbers in subsequent calculations) Report exponents as whole numbers (include the negative sign if necessary)
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter18: The Nucleus: A Chemist's View
Section: Chapter Questions
Problem 9Q
Related questions
Question
All boxes
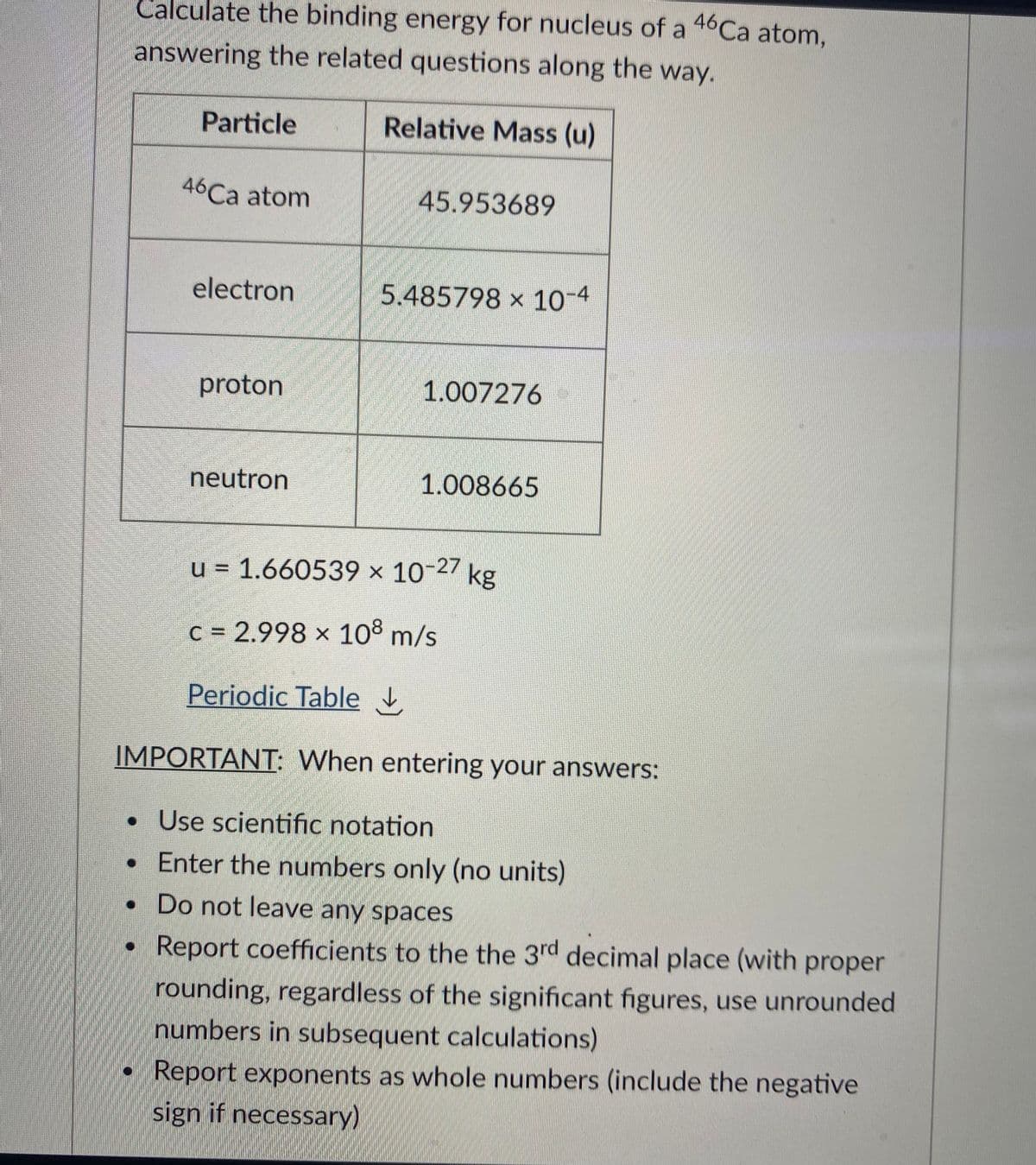
Transcribed Image Text:Calculate the binding energy for nucleus of a 46Ca atom,
answering the related questions along the way.
Particle
Relative Mass (u)
46Ca atom
45.953689
electron
5.485798 × 10-4
proton
1.007276
neutron
1.008665
u = 1.660539 x 10-27 kg
c = 2.998 x 10° m/s
Periodic Table
IMPORTANT: When entering your answers:
• Use scientific notation
• Enter the numbers only (no units)
• Do not leave any spaces
Report coefficients to the the 3rd decimal place (with proper
rounding, regardless of the significant figures, use unrounded
numbers in subsequent calculations)
Report exponents as whole numbers (include the negative
sign if necessary)
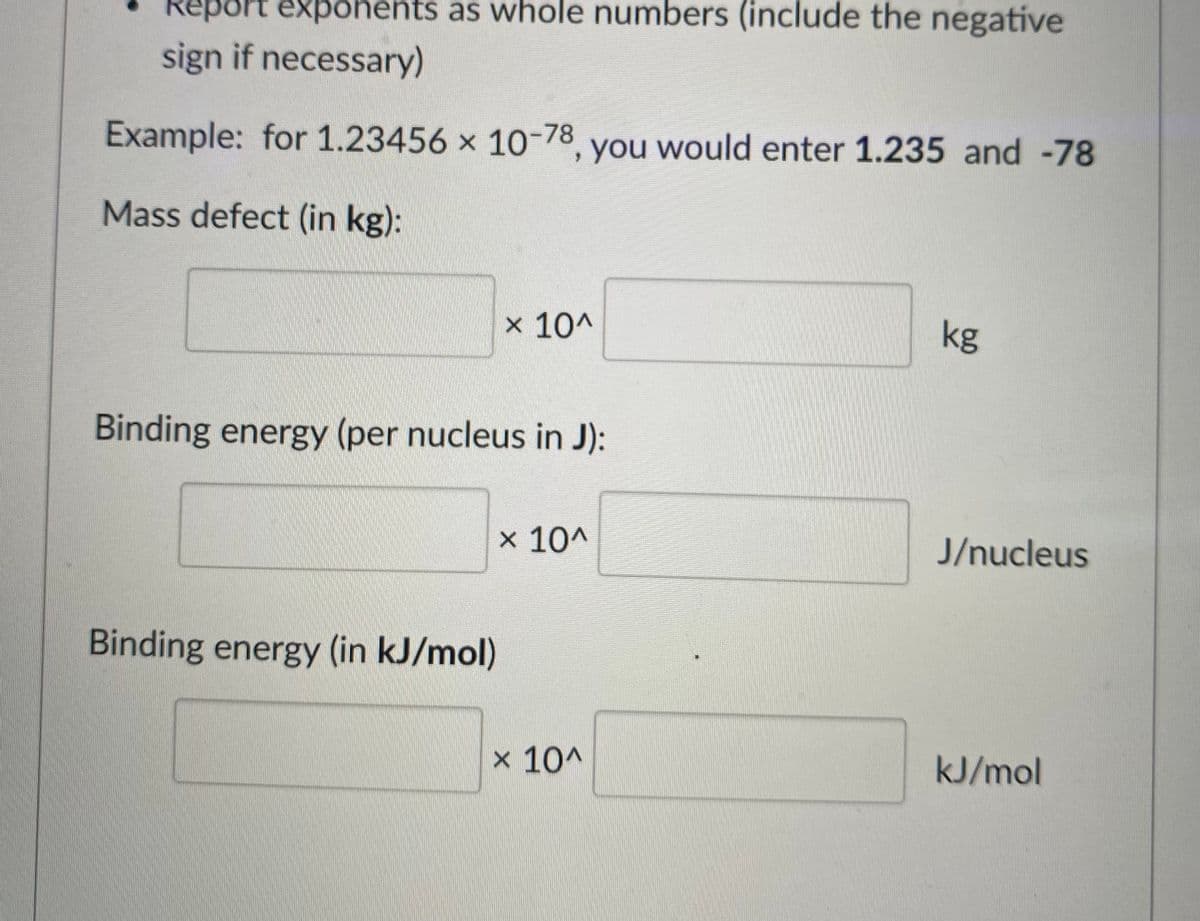
Transcribed Image Text:Report exponents as whole numbers (include the negative
sign if necessary)
Example: for 1.23456 x 10-78, you would enter 1.235 and -78
Mass defect (in kg):
x 10^
kg
Binding energy (per nucleus in J):
x 10^
J/nucleus
Binding energy (in kJ/mol)
x 10^
kJ/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning