Calculate the conductivity of 0.6400 Al2(SO4)3 in units of S/cm. Cation AM AM Anion (S/M-cm) (S/M-cm) H* 0.3496 OH 0.1991 Na* 0.05010 F 0.0554 K+ 0.07350 CI 0.07635 Lit 0.0387 Br 0.0781 Mg2+ 0.1060 NO3 0.07146 Ca2+ 0.1190 CIO, 0.0673 Ba2+ 0.1272 so,2- 0.1600 A13+ 0.183 PO43 0.207
Calculate the conductivity of 0.6400 Al2(SO4)3 in units of S/cm. Cation AM AM Anion (S/M-cm) (S/M-cm) H* 0.3496 OH 0.1991 Na* 0.05010 F 0.0554 K+ 0.07350 CI 0.07635 Lit 0.0387 Br 0.0781 Mg2+ 0.1060 NO3 0.07146 Ca2+ 0.1190 CIO, 0.0673 Ba2+ 0.1272 so,2- 0.1600 A13+ 0.183 PO43 0.207
Chapter32: Gas Chromatography
Section: Chapter Questions
Problem 32.7QAP
Related questions
Question
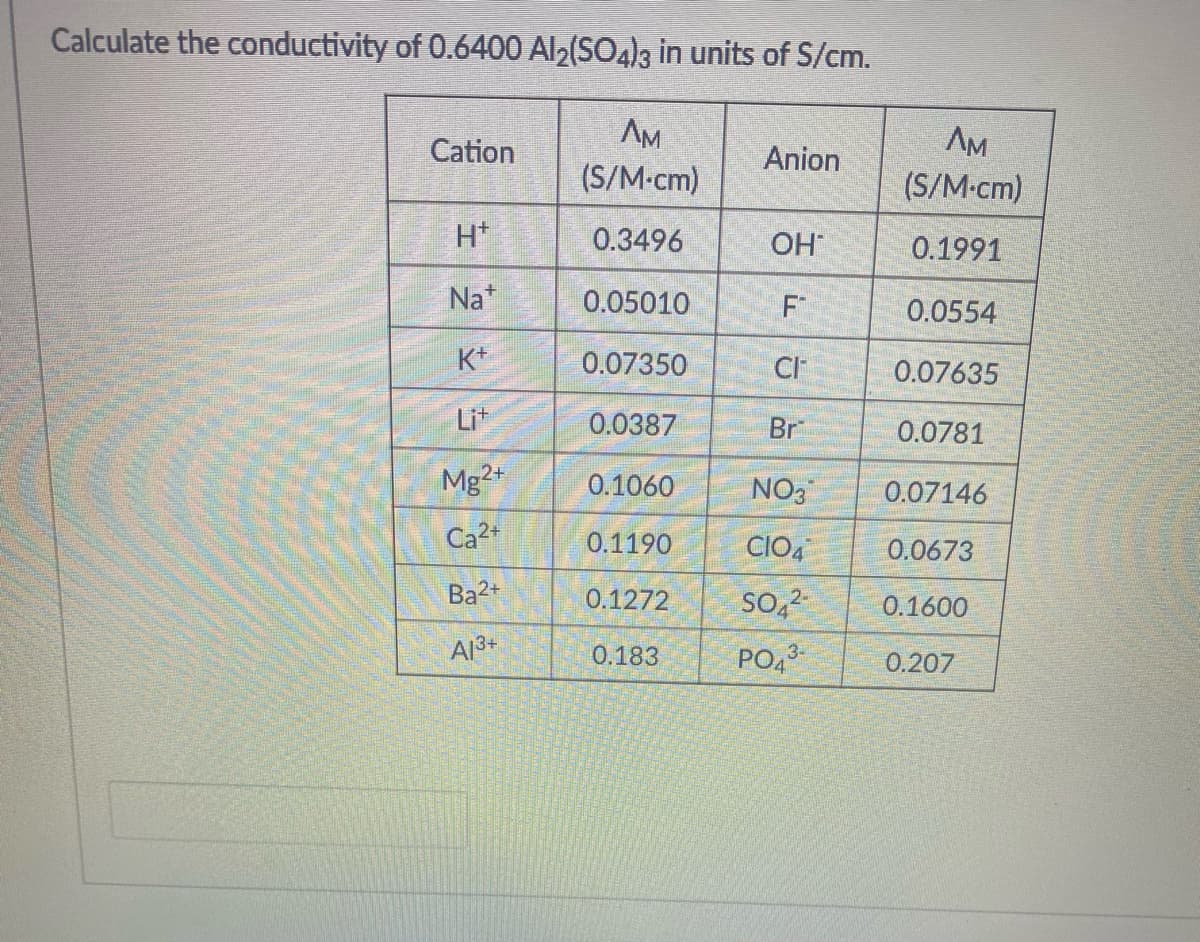
Transcribed Image Text:Calculate the conductivity of 0.6400 Al2(SO4)3 in units of S/cm.
AM
AM
(S/M-cm)
Cation
Anion
(S/M-cm)
H*
0.3496
OH
0.1991
Na*
0.05010
F
0.0554
K+
0.07350
CI
0.07635
Lit
0.0387
Br
0.0781
Mg2+
0.1060
NO3
0.07146
Ca2+
0.1190
CIO,
0.0673
Ba2+
0.1272
So,2
0.1600
A13+
PO4
0.183
3-
0.207
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

