Calculate the density of water using the data below: * Weight of beaker and water, g 44.5369 Weight of beaker, g 34.5802 Volume of water, mL 10 Temperature, °C 30 O 9.9567 g/mol 9.9567 g/mL O 0.9957 g/mol 0.9957 g/mL
Calculate the density of water using the data below: * Weight of beaker and water, g 44.5369 Weight of beaker, g 34.5802 Volume of water, mL 10 Temperature, °C 30 O 9.9567 g/mol 9.9567 g/mL O 0.9957 g/mol 0.9957 g/mL
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter2: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 2.16E: With reference to Joules apparatus inFigure2.6, assuminga massof 100.Kg ofwater about100L,a weight...
Related questions
Question
100%
good day maam/sir.. please do help me with this problem because we are going to orally solve virtually later. atleast I will be going to study some lessons related to our oral later at 2pm....
In return I promise to give helpful rating directly promise.
no need for solution. plss
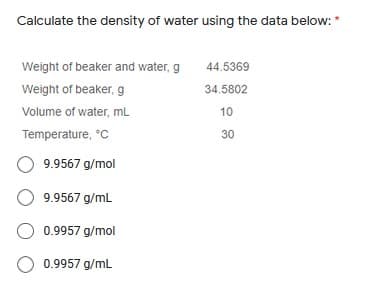
Transcribed Image Text:Calculate the density of water using the data below: *
Weight of beaker and water, g
44.5369
Weight of beaker, g
34.5802
Volume of water, mL
10
Temperature, °C
30
O 9.9567 g/mol
9.9567 g/mL
O 0.9957 g/mol
O 0.9957 g/mL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning