Calculate the lattice energy of AgCl(s) using the following thermodynamic data (all data is in kJ/mol). Note that the data given has been perturbed, so looking up the answer is probably not a good idea. Ag(s) AHsublimation = 265 kJ/mol Ag(g) Ionization energy = 711 kJ/mol Cl-CI(g) Bond energy = 223 kJ/mol CI(g) Electron affinity = -369 kJ/mol AgCl(s) AH°r=-147 kJ/mol kJ/mol Do you expect this value to be larger or smaller than the lattice energy of AgBr(s)?
Calculate the lattice energy of AgCl(s) using the following thermodynamic data (all data is in kJ/mol). Note that the data given has been perturbed, so looking up the answer is probably not a good idea. Ag(s) AHsublimation = 265 kJ/mol Ag(g) Ionization energy = 711 kJ/mol Cl-CI(g) Bond energy = 223 kJ/mol CI(g) Electron affinity = -369 kJ/mol AgCl(s) AH°r=-147 kJ/mol kJ/mol Do you expect this value to be larger or smaller than the lattice energy of AgBr(s)?
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter7: Chemical Bonding And Molecular Geometry
Section: Chapter Questions
Problem 84E: The lattice energy of KF is 794 kJ/mol, and the interionic distance is 269 pm. The Na—F distance in...
Related questions
Question
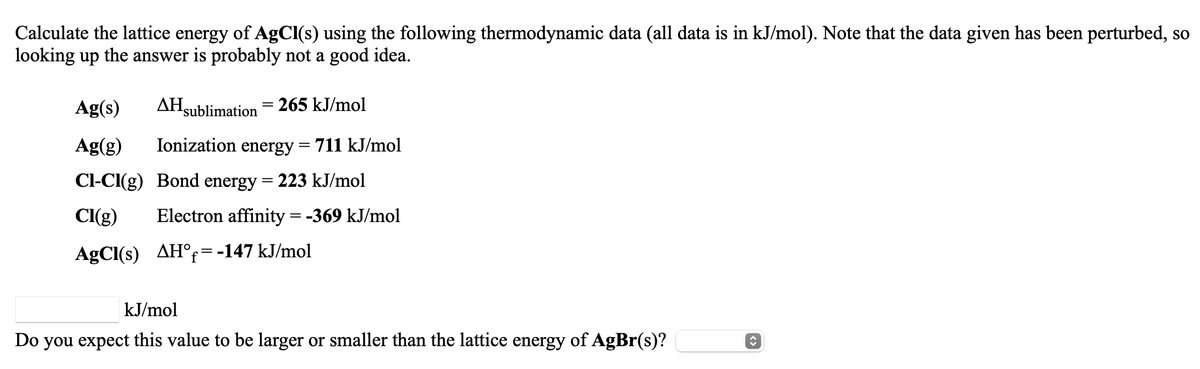
Transcribed Image Text:Calculate the lattice energy of AgCl(s) using the following thermodynamic data (all data is in kJ/mol). Note that the data given has been perturbed, so
looking up the answer is probably not a good idea.
Ag(s)
AHsublimation
265 kJ/mol
Ag(g)
Ionization energy
711 kJ/mol
Cl-CI(g) Bond energy = 223 kJ/mol
CI(g)
Electron affinity = -369 kJ/mol
AgCl(s) AH°=-147 kJ/mol
kJ/mol
Do you expect this value to be larger or smaller than the lattice energy of AgBr(s)?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning