Calculate the pressure exerted by 1.0 mol of hydrogen at 0°C in a vessel with volume of 5.0 dm3 using: a. The ideal gas law b. The Van der Waals equation (for constants see Table 1C.3, Resource section, p. 869) C. The Dieterici equation (for formula, refer to table of EOS in slide no. 63) d. The virial EOS up to 2nd virial coefficient (use constant from Table 1C.1, p. 868)
Calculate the pressure exerted by 1.0 mol of hydrogen at 0°C in a vessel with volume of 5.0 dm3 using: a. The ideal gas law b. The Van der Waals equation (for constants see Table 1C.3, Resource section, p. 869) C. The Dieterici equation (for formula, refer to table of EOS in slide no. 63) d. The virial EOS up to 2nd virial coefficient (use constant from Table 1C.1, p. 868)
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter1: Gases And The Zeroth Law Of Thermodynamics
Section: Chapter Questions
Problem 1.55E: The Berthelot equation of state for one mole of gas is p=RTV-b-aTV2 Where a and b are constants...
Related questions
Question
Answer only letter c and d.
Equation in letter c is provided in the picture.
Reference: Atkins' Physical Chemistry 11th ed.

Transcribed Image Text:Calculate the pressure exerted by 1.0 mol of hydrogen at 0°C in a vessel with volume of 5.0 dm³
using:
a. The ideal gas law
b. The Van der Waals equation (for constants see Table 1C.3, Resource section, p. 869)
C.
The Dieterici equation (for formula, refer to table of EOS in slide no. 63)
d. The virial EOS up to 2nd virial coefficient (use constant from Table 1C.1, p. 868)
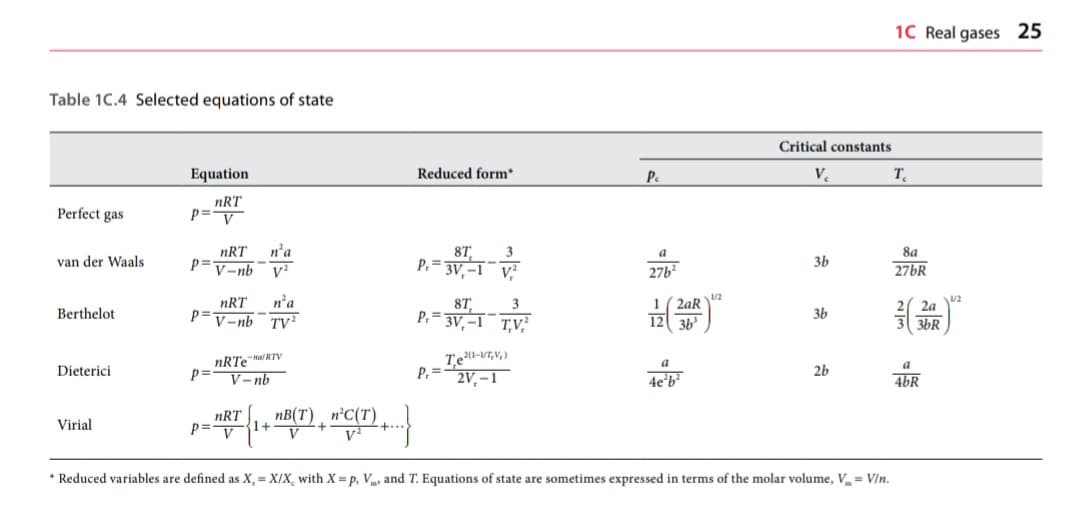
Transcribed Image Text:1C Real gases 25
Table 1C.4 Selected equations of state
Critical constants
Equation
Reduced form
Pe
V.
T.
Perfect gas
nRT
p=v
nRT
p=
n'a
V-nb
8T
3
8a
van der Waals
P.= 3V, –1 V
36
27b?
27BR
nRT
n'a
P=V-nb
1( 2aR
8T,
P. =
3V, –1¯ T,V
3
2a
Berthelot
36
TV?
3b
3bR
nRTea RTV
V– nb
a
a
Dieterici
P. =
2V, – 1
4e'b²
2b
46R
nRT
1+
n'C(T)
Virial
+
* Reduced variables are defined as X, = X/X, with X= p, V and T. Equations of state are sometimes expressed in terms of the molar volume, V = V/n.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning