Calculate the solubility at 25 °C of BaCro, in pure water and in a 0.0030 MBaCl, solution. You'll find K., data in the ALEKS Data tab. Round both of your answers to 2 significant digits. solubility in pure water: П solubility in 0.0030 M BaCl, solution:
Calculate the solubility at 25 °C of BaCro, in pure water and in a 0.0030 MBaCl, solution. You'll find K., data in the ALEKS Data tab. Round both of your answers to 2 significant digits. solubility in pure water: П solubility in 0.0030 M BaCl, solution:
Chapter10: Effect Of Electrolytes On Chemical Equilibria
Section: Chapter Questions
Problem 10.16QAP
Related questions
Question
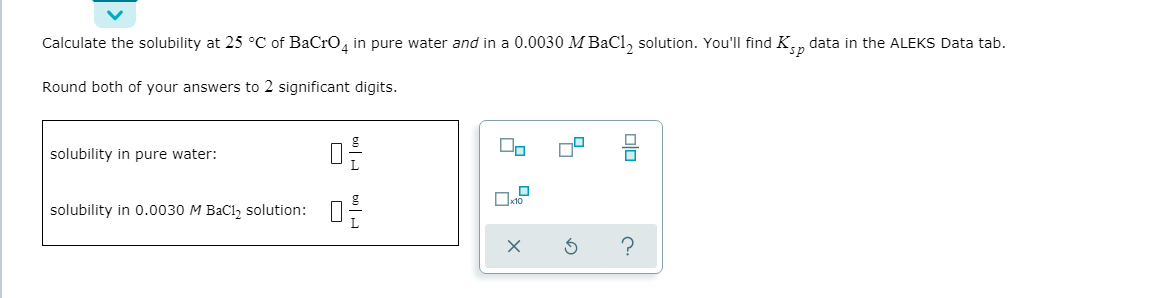
Transcribed Image Text:Calculate the solubility at 25 °C of BaCro, in pure water and in a 0.0030 MBaCl, solution. You'll find K., data in the ALEKS Data tab.
Round both of your answers to 2 significant digits.
solubility in pure water:
П
solubility in 0.0030 M BaCl, solution:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT