Calculate the standard enthalpy change for each of the following reactions. 1. Mg(OH)2(s)→MgO(s)+H2O(l) Express your answer in kilojoules to one decimal place. 2. N2O4(g)+4H2(g)→N2(g)+4H2O(g)
Calculate the standard enthalpy change for each of the following reactions. 1. Mg(OH)2(s)→MgO(s)+H2O(l) Express your answer in kilojoules to one decimal place. 2. N2O4(g)+4H2(g)→N2(g)+4H2O(g)
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter1: Introduction To Chemistry
Section: Chapter Questions
Problem 1.68QE
Related questions
Question
100%
Calculate the standard enthalpy change for each of the following reactions.
1. Mg(OH)2(s)→MgO(s)+H2O(l)
Express your answer in kilojoules to one decimal place.
2. N2O4(g)+4H2(g)→N2(g)+4H2O(g)
Express your answer in kilojoules to one decimal place.
3. SiCl4(l)+2H2O(l)→SiO2(s)+4HCl(g)
Express your answer in kilojoules to one decimal place.
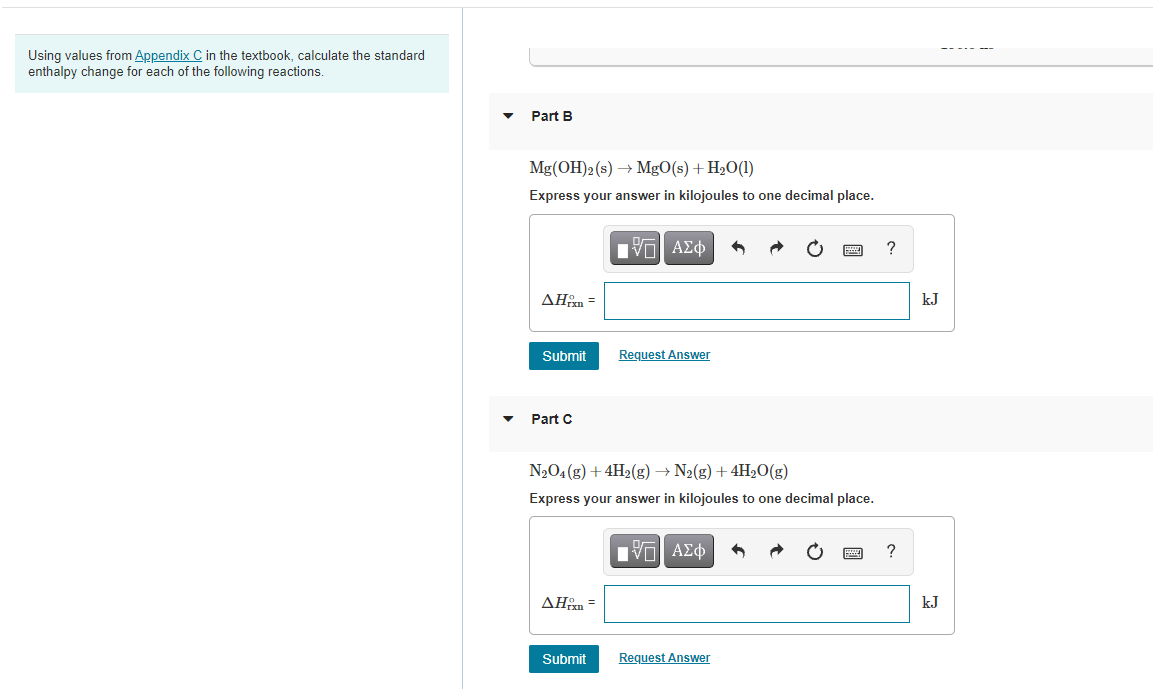
Transcribed Image Text:Using values from Appendix C in the textbook, calculate the standard
enthalpy change for each of the following reactions.
Part B
Mg(OH)2(s) → MgO(s) + H2O(1)
Express your answer in kilojoules to one decimal place.
?
ΔΗΡ
kJ
Submit
Request Answer
Part C
N2O4 (g) + 4H2(g) → N2(g) +4H2O(g)
Express your answer in kilojoules to one decimal place.
Πνα ΑΣφ
?
ΔΗ3
kJ
Submit
Request Answer
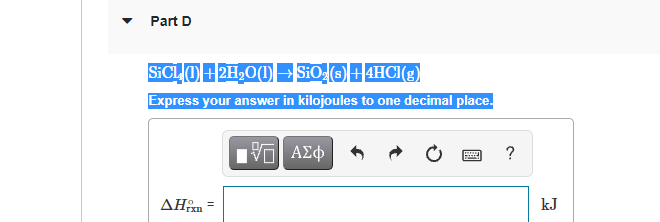
Transcribed Image Text:Part D
SICLO+2H,0(1) E SiO (O+4HCI(g)
Express your answer in kilojoules to one decimal place.
Πν ΑΣφ
?
ΔΗΡ
kJ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning