Calculating percent dissociation of a weak acid Calculate the percent dissociation of butanoic acid (C,H,CO,H) in a 1.4 mM aqueous solution of the stuff. You may find some useful data in the ALEKS Data resource. Round your answer to 2 significant digits. x10 [-] pK, of weak acids at 25°C Name Formula pKą1 pKą2 pКаз acetic acid CH3CO2H 4.756 benzoic acid CH5CO2H 4.204 butanoic acid Сзн,со2н 4.83 4-chlorobutanoic acid C3H,CICO,H 4.52 crotonic acid C3H5CO2H 4.69 охalic acid H2C2O4 1.25 3.81 phosphoric acid НзРО4 2.16 7.21 12.32 propionic acid C2H5CO2H 4.87 sulfuric acid H2SO4 strong acid 1.99 trimethylacetic acid C4H9CO2H 5.03
Calculating percent dissociation of a weak acid Calculate the percent dissociation of butanoic acid (C,H,CO,H) in a 1.4 mM aqueous solution of the stuff. You may find some useful data in the ALEKS Data resource. Round your answer to 2 significant digits. x10 [-] pK, of weak acids at 25°C Name Formula pKą1 pKą2 pКаз acetic acid CH3CO2H 4.756 benzoic acid CH5CO2H 4.204 butanoic acid Сзн,со2н 4.83 4-chlorobutanoic acid C3H,CICO,H 4.52 crotonic acid C3H5CO2H 4.69 охalic acid H2C2O4 1.25 3.81 phosphoric acid НзРО4 2.16 7.21 12.32 propionic acid C2H5CO2H 4.87 sulfuric acid H2SO4 strong acid 1.99 trimethylacetic acid C4H9CO2H 5.03
General, Organic, and Biological Chemistry
7th Edition
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:H. Stephen Stoker
Chapter10: Acids, Bases, And Salts
Section: Chapter Questions
Problem 10.45EP
Related questions
Question
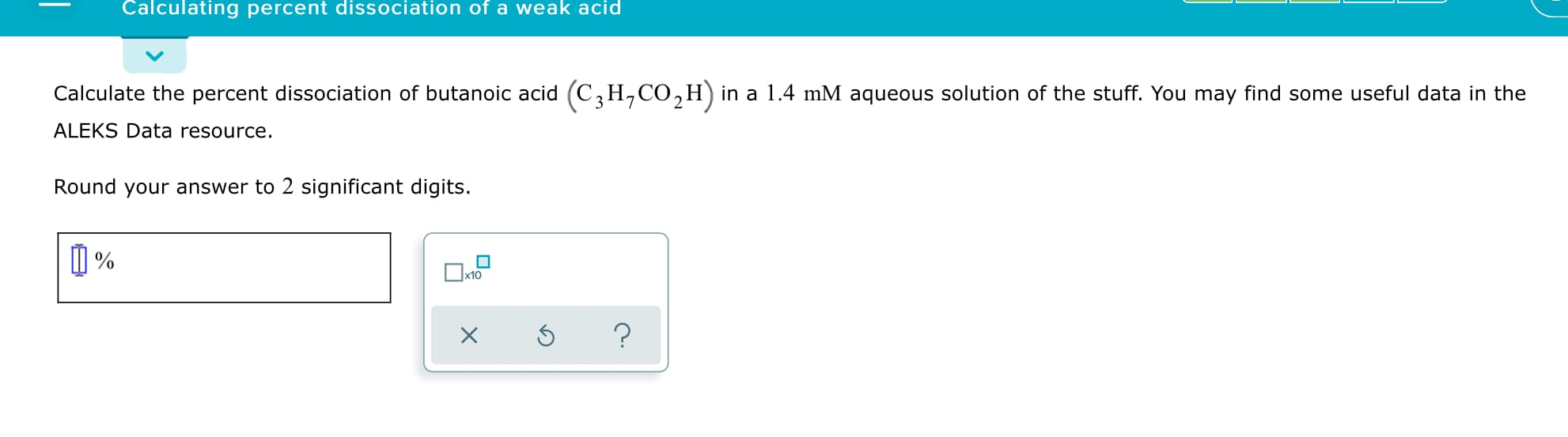
Transcribed Image Text:Calculating percent dissociation of a weak acid
Calculate the percent dissociation of butanoic acid (C,H,CO,H) in a 1.4 mM aqueous solution of the stuff. You may find some useful data in the
ALEKS Data resource.
Round your answer to 2 significant digits.
x10
![[-] pK, of weak acids at 25°C
Name
Formula
pKą1
pKą2
pКаз
acetic acid
CH3CO2H
4.756
benzoic acid
CH5CO2H
4.204
butanoic acid
Сзн,со2н
4.83
4-chlorobutanoic acid
C3H,CICO,H
4.52
crotonic acid
C3H5CO2H
4.69
охalic acid
H2C2O4
1.25
3.81
phosphoric acid
НзРО4
2.16
7.21
12.32
propionic acid
C2H5CO2H
4.87
sulfuric acid
H2SO4
strong acid
1.99
trimethylacetic acid
C4H9CO2H
5.03](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Feb286130-1ee5-4c42-a58a-074772cd1e39%2Fa6d75896-56d3-4676-98f7-53b4a9c6cf4d%2F5ytopd.jpeg&w=3840&q=75)
Transcribed Image Text:[-] pK, of weak acids at 25°C
Name
Formula
pKą1
pKą2
pКаз
acetic acid
CH3CO2H
4.756
benzoic acid
CH5CO2H
4.204
butanoic acid
Сзн,со2н
4.83
4-chlorobutanoic acid
C3H,CICO,H
4.52
crotonic acid
C3H5CO2H
4.69
охalic acid
H2C2O4
1.25
3.81
phosphoric acid
НзРО4
2.16
7.21
12.32
propionic acid
C2H5CO2H
4.87
sulfuric acid
H2SO4
strong acid
1.99
trimethylacetic acid
C4H9CO2H
5.03
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Recommended textbooks for you

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

